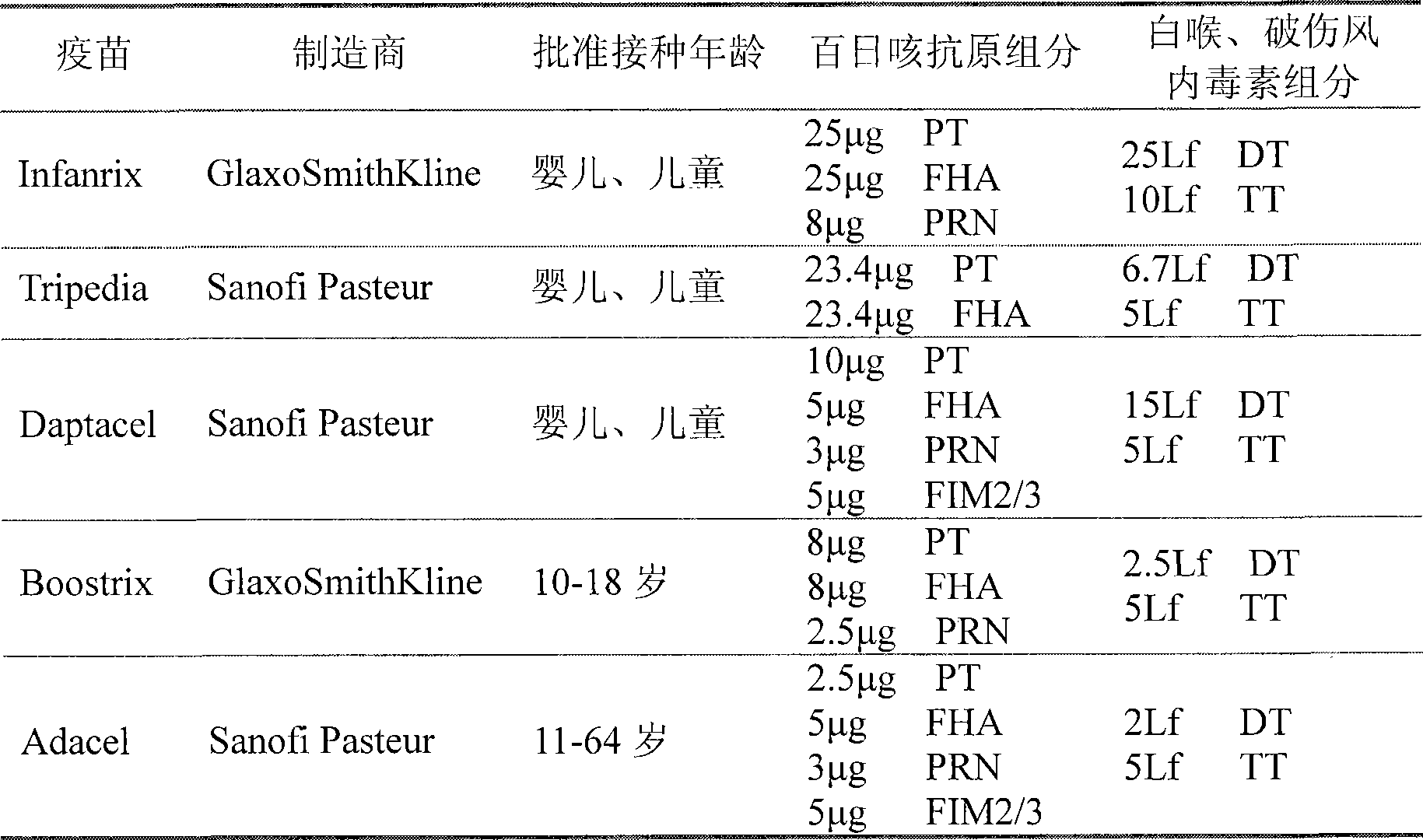
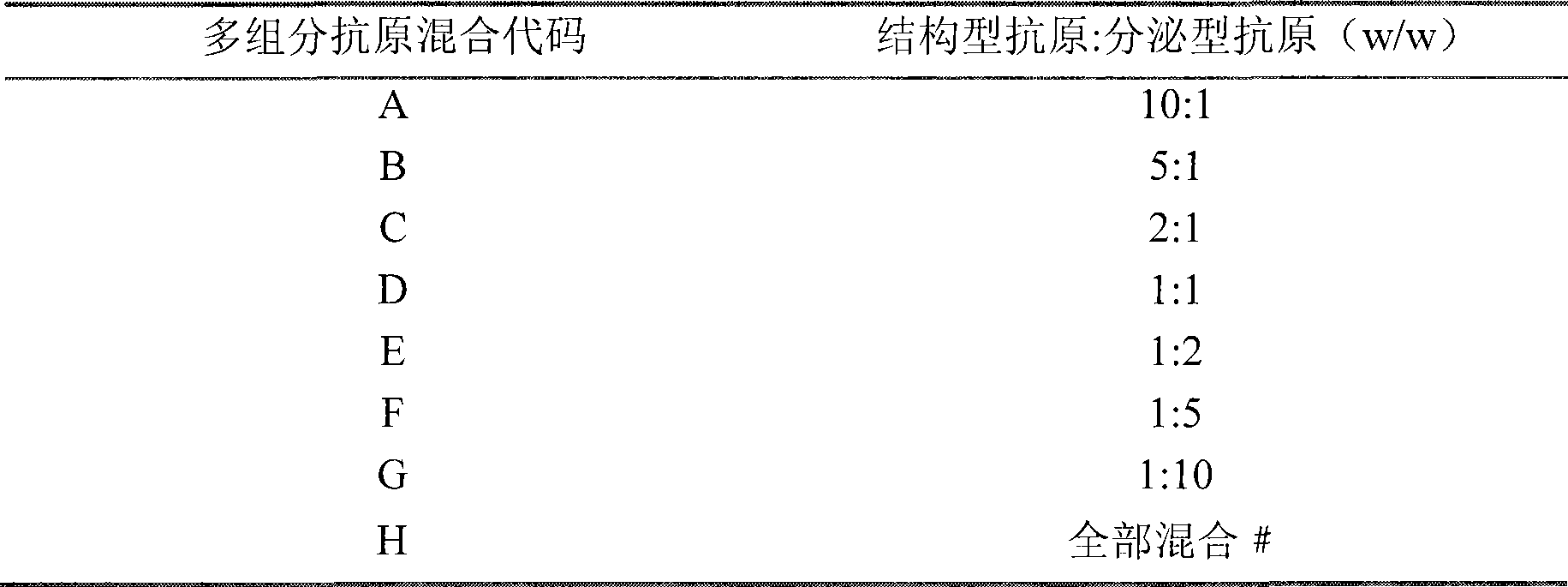
Multi-component antigen acellular pertussis vaccine and preparation method thereof
A pertussis, cell-free technology, applied in the direction of antibacterial drugs, bacterial antigen components, drug combinations, etc., can solve problems such as the inability to screen out the best aP vaccine, and the unresolved adverse reactions of wP vaccines.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Subculture of Bordetella pertussis strain and culture in fermenter
[0083] The strain of Bordetella pertussis used in the present invention is purchased from the Medical Bacteria Collection (CMCC), and its strain number is CMCC 58003, which is a phase I CS strain of pertussis. The full name of the Medical Bacteria Collection Center is the China Medical Microbiology Collection Management Center, and the Bordetella pertussis strains are preserved in the center's affiliated unit: National Institute for the Control of Pharmaceutical and Biological Products (NICPBP), Beijing. The agency produces, sells and sells Bordetella pertussis strains that are available for purchase by the public. Other suitable strains of Bordetella pertussis can also be used if desired, and the strains given here are merely illustrative of the invention.
[0084] Open the freeze-dried strain of Bordetella pertussis, inoculate it on Bao-ginger medium or other suitable medium, and culture it at 35-37°C...
Embodiment 2
[0086] Preparation of multicomponent cellular structural antigens of Bordetella pertussis
[0087] Add sodium deoxycholate phosphate buffer solution with a final concentration of 0.05-0.20% to the harvested bacterial cell sediment, shake at 2-8°C for 2-4 hours, then stir at 2-8°C for 18-24 hours to lyse cell. Add ammonium sulfate to a final concentration of 15-25%, place at 2-8°C for 5-7 days, centrifuge at 3,000-6,000 rpm for 30 minutes, and collect the precipitate. Add concentrated salt extract to the precipitate, stir at 2-8°C for 5-7 days, centrifuge at 9,000-18,000 rpm for 30-60 minutes, and collect the supernatant. Add ammonium sulfate to a final concentration of 25-35%, place at 2-8°C for 5-7 days, centrifuge at 4,000-9,000 rpm for 30-60 minutes, and collect the precipitate. Add concentrated salt extract to the precipitate, stir at 2-8°C for 3-5 days, then dialyze for 5-7 days, centrifuge at 10,000-30,000 rpm for 1-3 hours, and collect the supernatant. The supernatan...
Embodiment 3
[0089] Preparation of multicomponent cell-secreted antigen of Bordetella pertussis
[0090] Add ammonium sulfate to the harvested culture supernatant to a final concentration of 15-25%, place it at 2-8°C for 5-7 days, centrifuge at 3,000-6,000 rpm for 30 minutes, and collect the precipitate. Add concentrated salt extract to the precipitate, stir at 2-8°C for 5-7 days, centrifuge at 9,000-18,000 rpm for 30-60 minutes, and collect the supernatant. Add ammonium sulfate to a final concentration of 25-35%, place at 2-8°C for 5-7 days, centrifuge at 4,000-9,000 rpm for 30-60 minutes, and collect the precipitate. Add concentrated salt extract to the precipitate, stir at 2-8°C for 3-5 days, then dialyze for 5-7 days, centrifuge at 10,000-30,000 rpm for 1-3 hours, and collect the supernatant. The supernatant is purified by 10-30% sucrose density gradient centrifugation, centrifuged at 20,000-35,000 rpm for 16-24 hours, and the original antigen solution is collected. Add glutaraldehyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com