Acellular pertussis vaccine detoxified with hydrogen peroxide and preparation method thereof
A technology of hydrogen peroxide and whooping cough, applied in chemical instruments and methods, antibacterial drugs, bacterial antigen components, etc., can solve the problems of difficult sterilization and filtration, complicated reaction, lack of anti-aggregation agents, etc., and achieve good immunogenicity, The effect of high antigen recovery rate and broad-spectrum antigenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
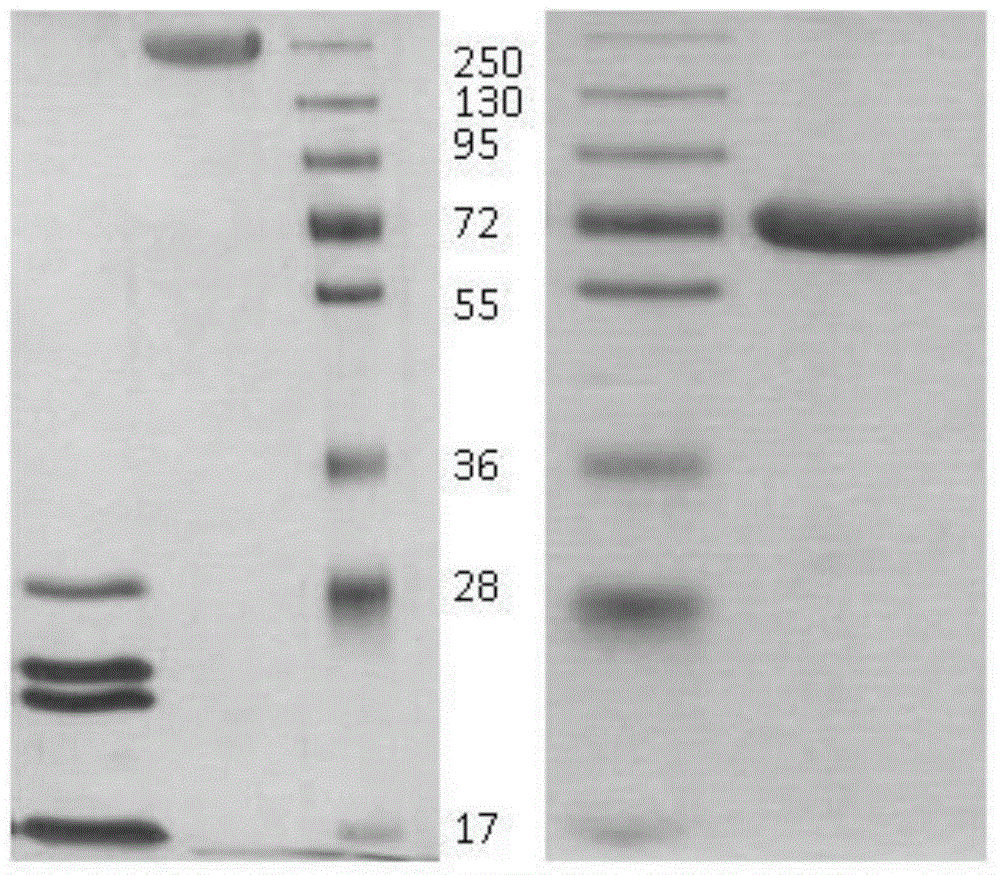
[0069] Example 1: Acquisition of pertussis antigens
[0070] After opening the pertussis phase I strain (No. 58003) purchased from the China Institute for the Control of Pharmaceutical and Biological Products, it was inoculated on the modified bag-ginger medium, and cultivated at 35 to 37 ° C for 70 hours to 72 hours. The second generation and the third generation were inoculated on activated carbon semi-synthetic solid medium, cultured at 35-37°C for 23-24 hours, and then inoculated on FMCL (Frohlich modified cyclodextrin liquid) medium or S-S (Stainer-Scholte) medium, at 35-37 Cultivated for 24 hours to 48 hours for production.
[0071] The static culture method or the fermenter culture method can be used, the temperature is 35.5-36.0 ℃, and the culture process should be sampled for pure bacteria inspection.
[0072] Cultures were harvested after the logarithmic growth phase or before the stationary phase.
[0073] Continuous flow centrifugation was used to separate the cu...
Embodiment 2
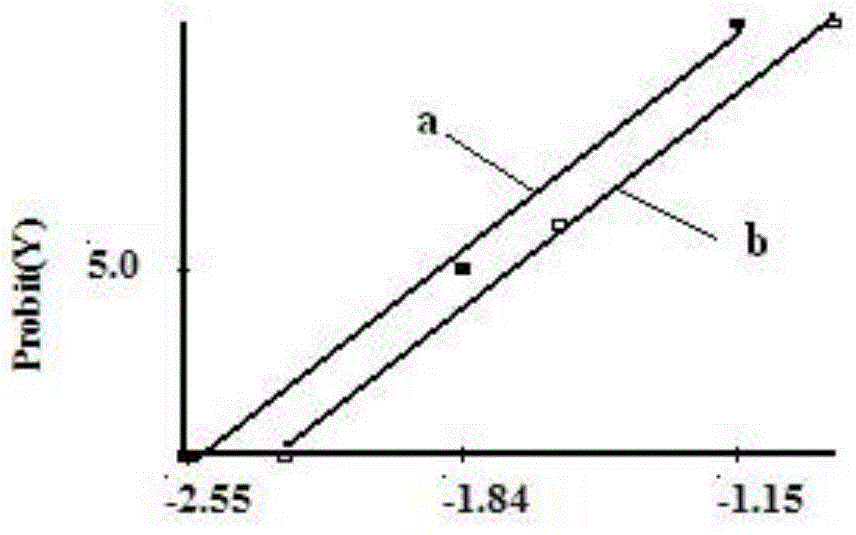
[0101] Example 2: Detoxification of PT antigens
[0102] a) The PT antigen purified in Example 1 was replaced by phosphate buffer by means of dialysis or ultrafiltration, with a concentration of 100 mmol / L and a pH of 8.0.
[0103] b) Dilute the antigen after replacing the buffer system in step a to 250 μg / ml.
[0104] c) Add glycerol and urea to the antigen diluted in step b so that the final concentration of glycerol reaches 30% (V / V) and the final concentration of urea reaches 2 mol / L.
[0105] d) After step c, add hydrogen peroxide with a final concentration of 1% (W / V) to adjust the pH to 8.0.
[0106] e) The detoxification temperature is 37°C, and the detoxification time is 4 hours.
[0107] f) Remove hydrogen peroxide by ultrafiltration to terminate detoxification.
[0108] g) After terminating the detoxification in step f, filter with a 0.22 μm filter membrane to obtain the PT stock solution after detoxification.
[0109] The test results of the PT stock solution a...
Embodiment 3
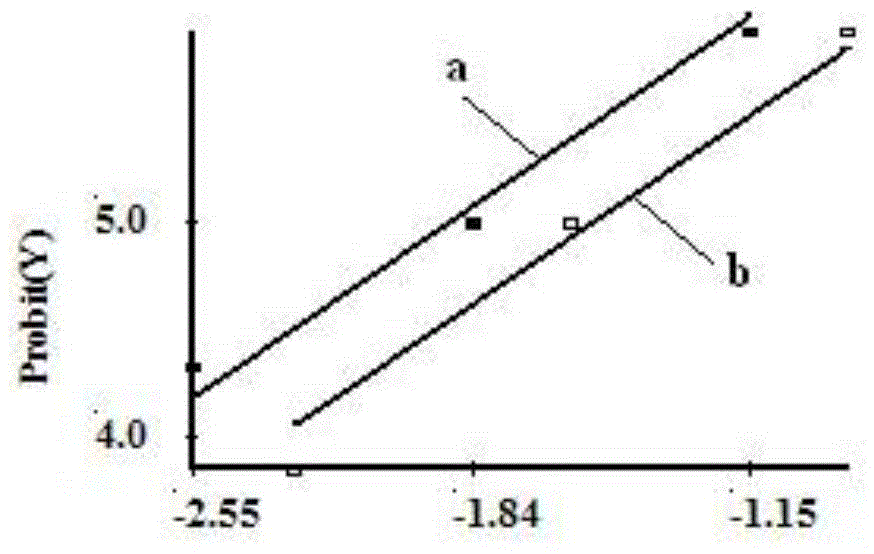
[0112] Example 3: Detoxification of FHA antigens
[0113] a) Replace the purified FHA antigen with phosphate buffer by means of dialysis or ultrafiltration, with a concentration of 100 mmol / L and a pH of 8.0.
[0114] b) Dilute the antigen after replacing the buffer system in step a to 250 μg / ml.
[0115] c) Add glycerol and urea to the antigen diluted in step b so that the final concentration of glycerol reaches 30% (V / V) and the final concentration of urea reaches 2 mol / L.
[0116] d) After step c, add hydrogen peroxide with a final concentration of 0.5% (W / V) to adjust the pH to 8.0.
[0117] e) The detoxification temperature is 37°C, and the detoxification time is 2 hours.
[0118] f) Remove hydrogen peroxide by ultrafiltration to terminate detoxification.
[0119] g) After terminating the detoxification in step f, filter with a 0.22 μm filter membrane to obtain the detoxified FHA stock solution.
[0120] The test results of FHA stock solution after detoxification are ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com