Monoclone antibody of ARD1 and application thereof
A monoclonal antibody and antibody technology, applied in the field of immunology, can solve the problems that cannot be used to detect ARD1
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
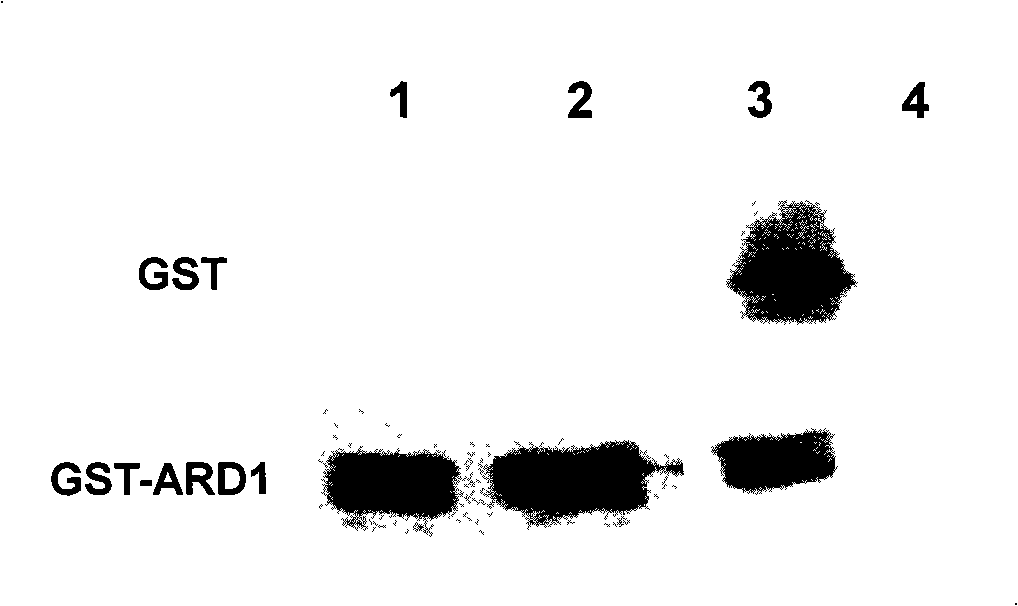
[0059] Embodiment 1 Preparation of anti-human ARD1 monoclonal antibody
[0060] 1. Preparation of ARD1 antigen
[0061] The 5' end sense primer 5'-ATTGGATCCTCGCCACCATGAACATCCGCAATGCG-3' (SEQ ID No.1) and the 3' end antisense primer 5'-AATGAATTCTAGGAGG CTGAGTCGGAG-3' (SEQ ID No.2) (primers were synthesized by Shanghai Sangon Bioengineering Co., Ltd.), and BamH I and EcoR I restriction sites were introduced at both ends of the primers. The total RNA of human colon cancer cell LoVo (ATCC number: CCL-229) was extracted with Trizol (purchased from Invitrogen Company), reverse-transcribed into cDNA, and the ARD1 cDNA fragment was amplified by PCR using the above primers, and the product length was 712bp.
[0062] The PCR product and the pGEX-5X-3 vector (purchased from Amersham Biosciences) were digested with BamH I and EcoR I respectively and ligated. The ligated product was transformed into E. coli BL21 strain and a single clone was picked for enzyme digestion identification , a...
Embodiment 2
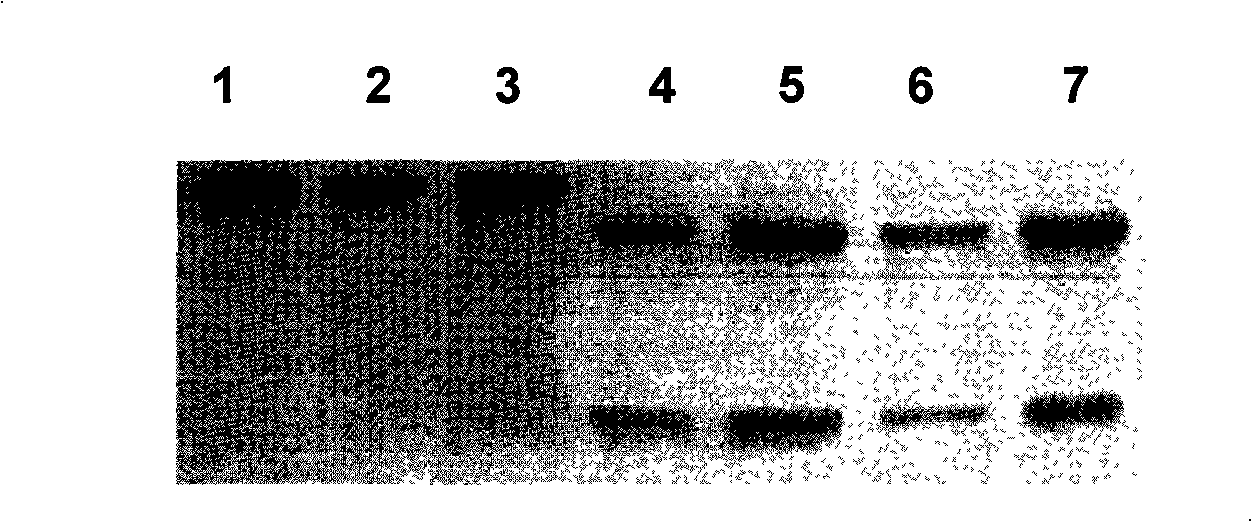
[0066] Embodiment 2 identifies the purity of 14D4 antibody or 10C12 antibody
[0067] The subtypes of 14D4 antibody or 10C12 antibody were identified by conventional ELISA method and confirmed to be IgG2a and IgG1 respectively. Therefore, Pro.A Sepharose-4B column was selected for purification, and its purity was identified by 12% SDS-PAGE electrophoresis. see results figure 1 , showing that the 14D4 antibody or 10C12 antibody has high purity and no bands.
Embodiment 3
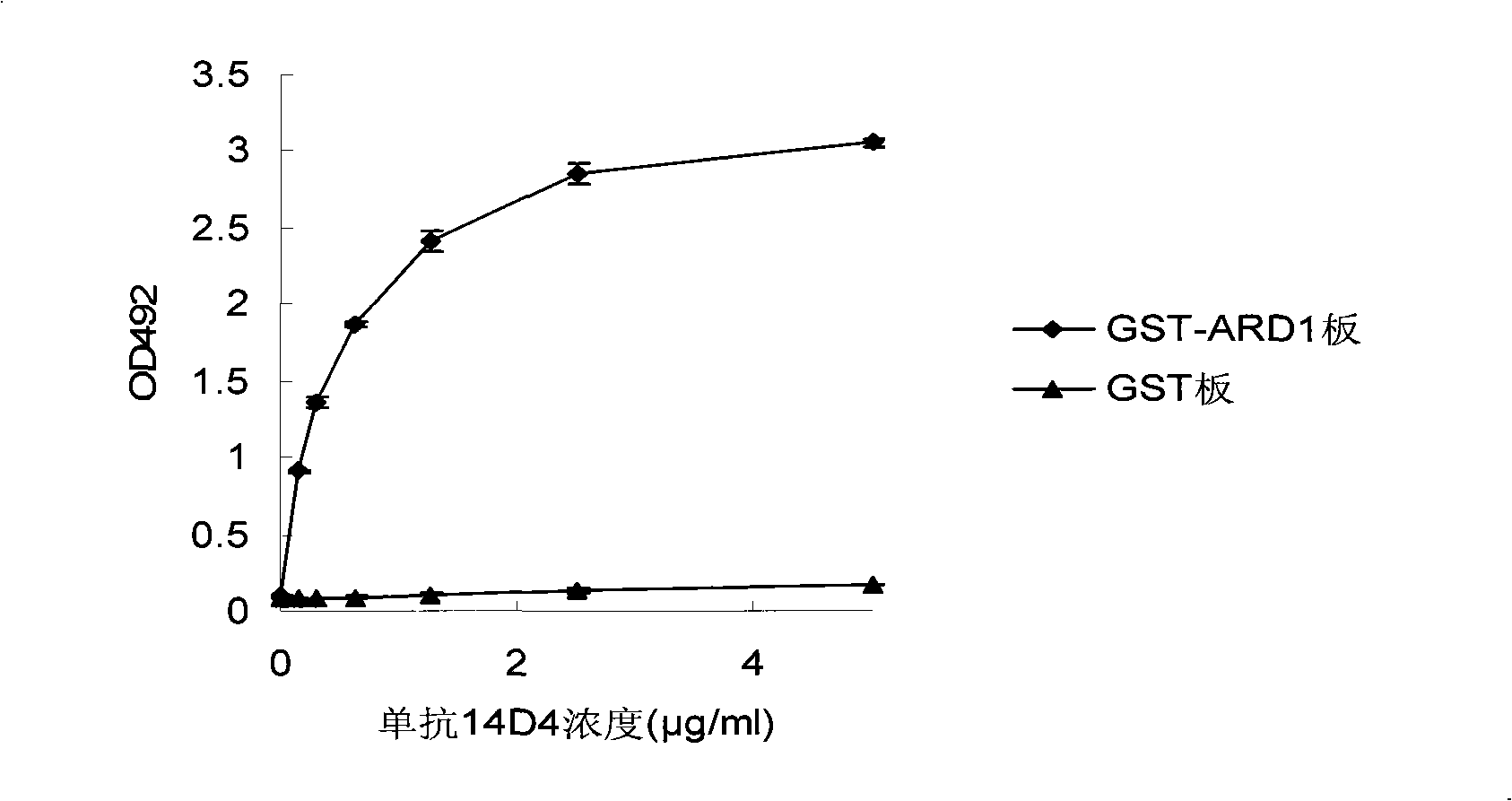
[0068] Example 3 Determination of the titer of 14D4 antibody
[0069] The purified 14D4 antibody was dialyzed with PBS buffer, and the concentration of the antibody was measured at 280 nm by an ultraviolet spectrophotometer.
[0070] 1. Add 14D4 antibody at concentrations of 0, 0.18, 0.37, 0.625, 1.25, 2.5, and 5 μg / ml to the microtiter plate coated with GST-ARD1 protein. And the same concentration of 14D4 antibody was added to the GST protein-coated microtiter plate as a negative control. Incubate for 1 hour at room temperature.
[0071] 2. Wash the reaction wells 3 times with 0.05% Tween-20 / PBS, and 2 times with PBS.
[0072] 3. Add horseradish peroxidase-labeled goat anti-mouse IgG antibody and incubate at room temperature for 1 hour.
[0073] 4. Wash the reaction wells 3 times with 0.05% Tween-20 / PBS, and 2 times with PBS.
[0074] 5. Add horseradish peroxidase substrate OPD to the reaction well, after developing color at room temperature for 30 minutes, add stop solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com