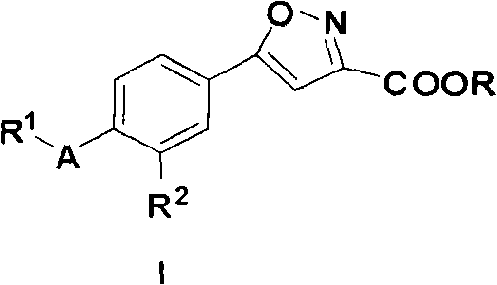
5-substituted phenyl-3-isoxazole carboxylic acid and ester compounds, compositions and preparation method thereof
A technology of ethyl isoxazolecarboxylate and isoxazolecarboxylate, which is applied in the field of medical technology, can solve the problem of not finding a preparation method for 5-substituted phenyl-3-isoxazolecarboxylate and its ester compound, etc., and achieves Effect of prevention of hyperuricemia and gout, simple and easy preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
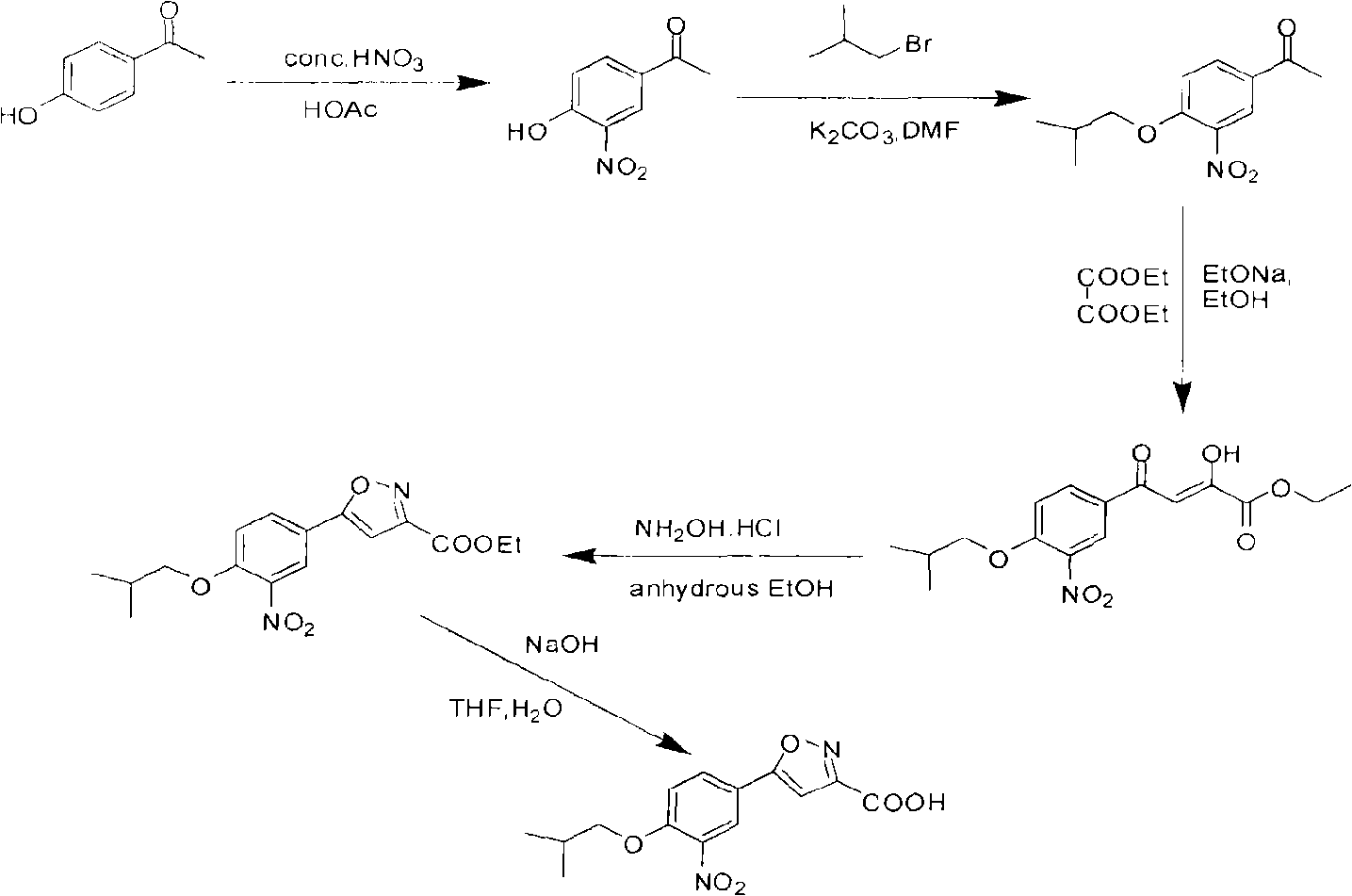
[0062] Preparation of 5-(3-nitro-4-isobutoxy)phenyl-3-isoxazolecarboxylic acid
[0063] The title compound was prepared according to the method of the following scheme
[0064]
[0065] (1) Preparation of 3-nitro-4-hydroxyacetophenone
[0066] Dissolve p-hydroxyacetophenone (15.00g, 110.00mmol) in glacial acetic acid (40mL), stir at 45°C, slowly add concentrated nitric acid (10.5mL, 110.00mmol) dropwise, control the reaction temperature at 50°C-60°C, After dropping, continue to stir for 0.5h. The reaction solution was poured into a mixture of ice and water, a yellow solid precipitated out, filtered with suction, dried naturally at room temperature, and then recrystallized with absolute ethanol to obtain 10.50 g of yellow crystals, yield 52.6%, m.p.131.0-132.6°C.
[0067] (2) Preparation of 3-nitro-4-isobutoxyacetophenone
[0068] Add 3-nitro-4-hydroxyacetophenone (15.80 g, 87.30 mmol), anhydrous potassium carbonate (42.20 g, 306.00 mmol), DMF (150.0 mL), PEG-400 (4.0 mL)...
Embodiment 2
[0082] Preparation of 5-(3-nitro-4-benzyloxy)phenyl-3-isoxazolecarboxylic acid
[0083] The title compound was prepared according to the method of the following scheme
[0084]
[0085] (1) Preparation of 3-nitro-4-hydroxyacetophenone
[0086] Concrete operation refers to (1) in embodiment 1
[0087] (2) Preparation of 3-nitro-4-benzyloxyacetophenone
[0088] In a 250ml round bottom flask was added 3-nitro-4-hydroxyacetophenone (5.00g, 27.60mmol), anhydrous potassium carbonate (11.40g, 82.90mmol), potassium iodide (0.27g, 1.38mmol) and DMF (50mL ), after stirring at 65°C for 20min, benzyl chloride (6.4mL, 55.20mmol) was added, and the reaction was continued at this temperature for 3h. Then the reaction solution was poured into water (300mL), the solid was precipitated, and the crude product was obtained by suction filtration, which was recrystallized from ethyl acetate to obtain 5.00g of light yellow crystals, yield 66.8%, m.p.132.7-134.7°C.
[0089] (3) Preparation of ...
Embodiment 3
[0099] Preparation of 5-[3-nitro-4-(4-methyl)benzyloxy]phenyl-3-isoxazolecarboxylic acid
[0100] The title compound was prepared according to the method of the following scheme
[0101]
[0102] (1) Preparation of 3-nitro-4-hydroxyacetophenone
[0103] Concrete operation refers to (1) in embodiment 1
[0104] (2) Preparation of 3-nitro-4-(4-methyl)benzyloxyacetophenone
[0105] In a 250ml round bottom flask were added 3-nitro-4-hydroxyacetophenone (5.00g, 27.60mmol), anhydrous potassium carbonate (11.40g, 82.90mmol), potassium iodide (0.30g, 1.38mmol) and DMF (50mL ), after stirring at 65°C for 20min, 4-methylbenzyl chloride (7.80g, 55.20mmol) was added, and the reaction was continued at this temperature for 3h. Then the reaction liquid was poured into water (300mL), the solid was precipitated, and the crude product was obtained by suction filtration. Recrystallized from ethyl acetate to obtain 5.20g of yellow crystals, yield 66.0%, m.p.108.8-110.8°C.
[0106] 1 H-NMR...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap