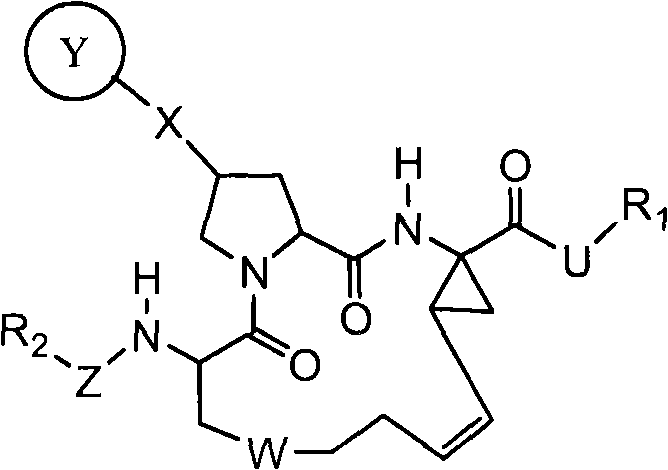
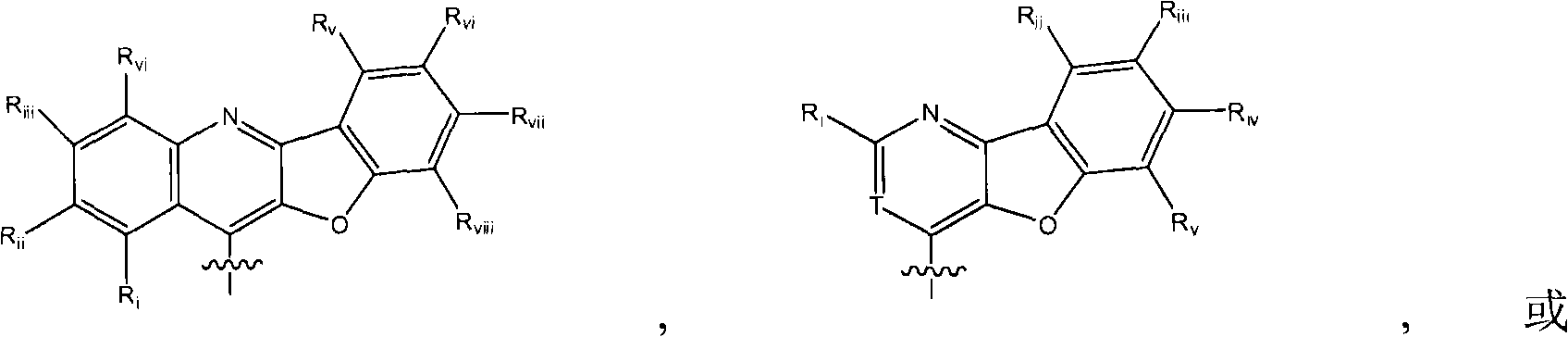
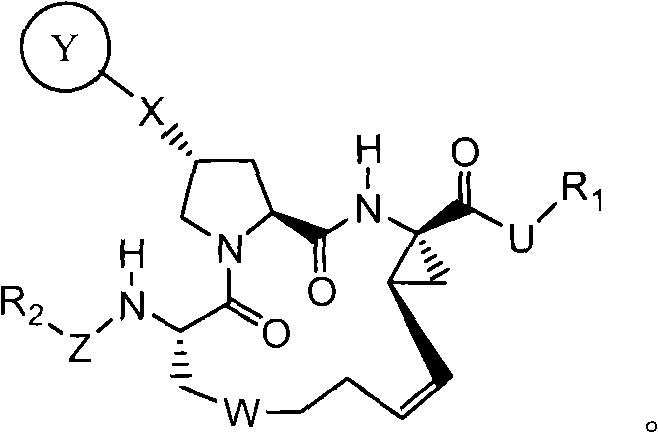
Hcv protease inhibitors
A technology of inhibitors and compounds, applied in antiviral agents, medical preparations containing active ingredients, cyclic peptide components, etc., can solve problems such as side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 : {4-cyclopropanesulfonylaminocarbonyl-2,15-dioxo-18-[2-(4-trifluoromethyl-phenyl)-benzo[4,5]furo(furo)[3 ,2-d]pyrimidin-4-yloxy]-3,16-diazatricyclo[14.3.0.04,6]nonadec-7-en-14-yl}-carbamic acid cyclopentyl ester (compound 1) Synthesis of
[0056] First, compound 1-3 was prepared from commercially available ethyl 1-tert-butoxycarbonylamino-2-vinyl-cyclopropanecarboxylate via the following route:
[0057]
[0058] To a solution of ethyl 1-tert-butoxycarbonylamino-2-vinyl-cyclopropanecarboxylate (0.34 g, 1.3 mmol) in THF (5 mL) and methanol (5 mL) was added LiOH (0.13 g , 5.3 mmol) in water (1.4 mL). After stirring overnight at room temperature, the reaction was quenched with 10% HCl (2 mL) and the solvent was removed in vacuo. The obtained solid powder was washed with water (10 mL) to obtain compound I-1 (0.27 g, 90%). MS m / z249.9 (M + +23); 1 H NMR (CDCl 3 )δ10.35(brs, 1H), 5.84-5.71(m, 1H), 5.29(d, J=17.4Hz, 1H), 5.12(d, J=10.2Hz, 1H), 2.23-2.14(m,...
Embodiment 2
[0072] Example 2 : {4-cyclopropanesulfonylaminocarbonyl-2,15-dioxo-18-[2-(4-trifluoromethyl-phenyl)-benzo[4,5]furfurylfuro[3, 2-d]pyrimidin-4-yloxy]-3,16-diaza-tricyclo[14.3.0.04,6]nonadec-7-en-14-yl}-carbamic acid cyclopentyl ester (compound 2) Synthesis of
[0073] Compound 2 was prepared by the route shown below:
[0074]
[0075] Add compound I-11 (0.11 g, 0.14 mmol) in 5 mL CH at room temperature 2 Cl 2 The solution in was added with 4N HCl in dioxane (2 mL) for 4 hours. Removal of HCl, dioxane and CH by evaporation 2 Cl 2 , the crude product compound I-12 was obtained, and the crude product I-12 was directly used in the next step without further purification.
[0076] Dissolve the crude product I-12 in 2 mL of acrylonitrile, then add saturated NaHCO 3 aqueous solution (1 mL). After stirring for 10 minutes, cyclopentyl chloroformate (0.02 g, 0.15 mmol) was added to the reaction mixture at room temperature. The reaction mixture was stirred for an additional 2...
Embodiment 3-52
[0078] Example 3-52 : Synthesis of Compound 3-52
[0079] Each of Compounds 3-52 was prepared in a manner similar to that described in Examples 1 and 2.
[0080] Compound 3: MS: m / z 857.3 (M + +1); 1 H NMR (CDCl 3 ) 10.42(s, 1H), 8.41(d, 2H), 7.97(s, 1H), 7.51-7.35(m, 2H), 7.20(s, 1H), 7.02(d, 2H), 6.10(s, 1H) , 5.68(q, 1H), 5.16(d, 1H), 4.96(dd, 1H), 4.75(dd, 1H), 4.65(d, 1H), 4.34-4.07(m, 2H), 3.90(s, 3H ), 2.97-2.50 (m, 3H), 2.51 (s, 3H), 2.30 (q, 1H), 2.05-0.81 (m, 25H).
[0081] Compound 4: MS: m / z 869.3 (M + +1);
[0082] Compound 5: MS: m / z 803.3 (M + +1); 1 H NMR (CDCl 3 ) 10.31(s, 1H), 8.29(d, 1H), 7.67-7.54(m, 3H), 7.44(dd, 1H), 7.27(d, 1H), 7.03(s, 1H), 6.58(dd, 1H) , 6.09(s, 1H), 5.69(q, 1H), 5.05(d, 1H), 4.97(dd, 1H), 4.74(dd, 1H), 4.63(d, 1H), 4.26-4.04(m, 2H ), 2.95-2.20 (m, 4H), 1.95-1.15 (m, 14H), 1.08 (s, 9H), 0.98-0.81 (m, 2H).
[0083] Compound 6: MS: m / z 815.3 (M + +1); 1 H NMR (CDCl 3 )δ10.38(s, 1H), 8.29(d, 1H), 7.67-7.38(m, 4H), 7.2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com