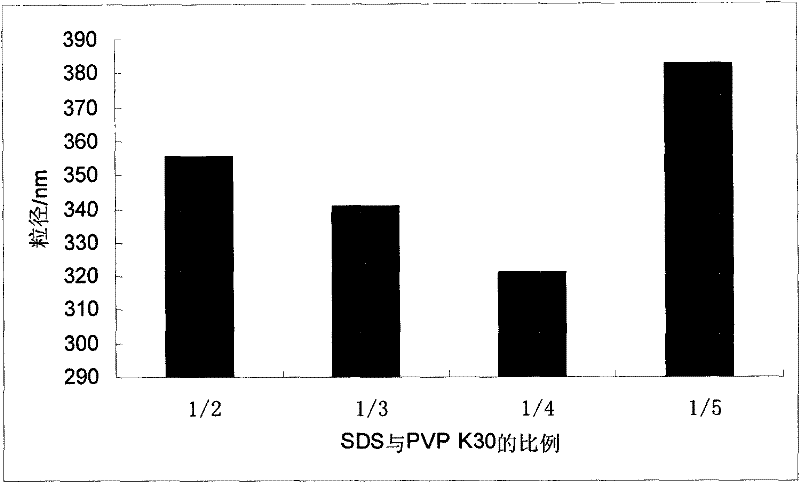
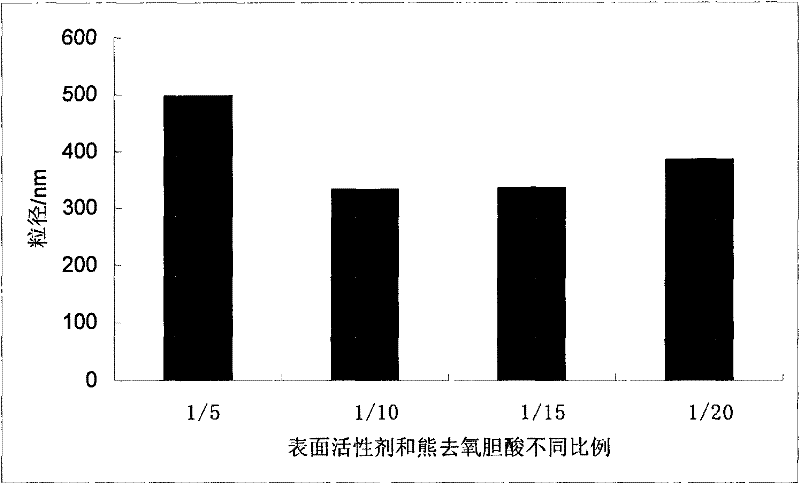
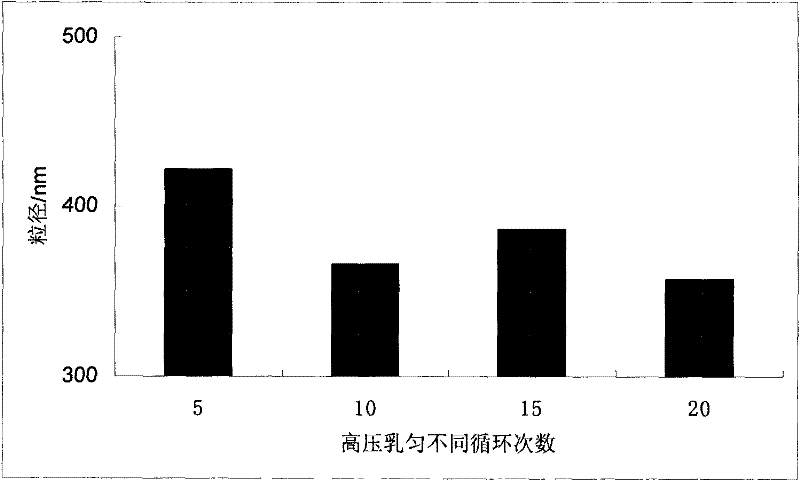
Ursodeoxycholic acid nano suspension and preparation method thereof
A technology of ursodeoxycholic acid and nanosuspension, which can be used in pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve problems such as shelving, increase absorption opportunities, improve Dissolution, the effect of solving physical stability problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Weigh 2 g of ursodeoxycholic acid raw material, 0.16 g of PVP K30 and 0.04 g of SDS, add 40 mL of double distilled water, stir well, ultrasonically emulsify with 8000 rpm probe for 2 min; The preparation conditions were 2 cycles at 200 bar, 2 cycles at 500 bar, and 20 cycles at 1500 bar to obtain a milky white nanosuspension with a measured particle size of 356 nm, PI 0.13, and zeta potential of -48 mv.
Embodiment 2
[0034] Weigh 2 g of ursodeoxycholic acid raw material, 0.16 g of PVP K30 and 0.04 g of SDS, add 40 mL of double distilled water, stir well, ultrasonically emulsify with 8000 rpm probe for 2 min; The preparation conditions were 2 cycles at 200 bar, 2 cycles at 500 bar, 2 cycles at 1000 bar, and 10 cycles at 1500 bar to obtain a milky white nanosuspension with a measured particle size of 380 nm, PI 0.15, and zeta potential of -45 mv.
Embodiment 3
[0036]Weigh 2 g of ursodeoxycholic acid raw material, 0.1 g of PVP K30 and 0.1 g of SDS, add 40 mL of double distilled water, stir well, ultrasonically emulsify with 8000 rpm probe for 2 min; The preparation conditions were 2 cycles at 200 bar, 2 cycles at 500 bar, and 20 cycles at 1500 bar to obtain a milky white nanosuspension with a measured particle size of 420 nm, PI 0.17, and zeta potential of -35 mv.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com