Method for controlling quality of euphorbia kansui decoction pieces
A decoction piece and content technology, which is applied in the field of quality control of traditional Chinese medicine kansui decoction pieces, can solve the problems of restricting research and development of kansui decoction pieces, difficult to ensure the safety and effectiveness of kansui decoction pieces, and achieves the effect of easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
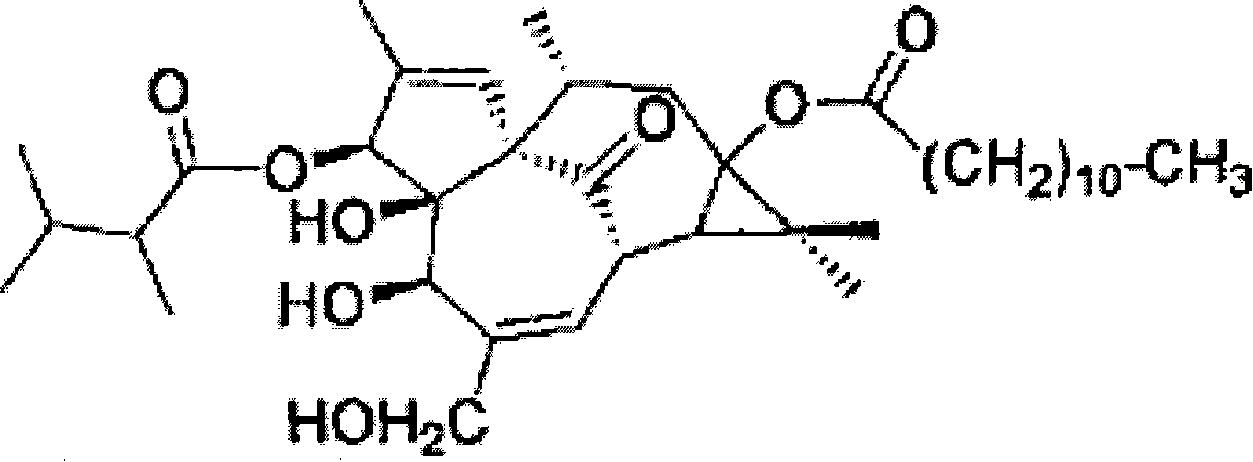
[0018] (1) Take 8 batches of raw Gansui decoction pieces purchased from Shanghai, Anhui, Sichuan, Hebei, and Gansu, grind them into fine powder, weigh about 2g, weigh them accurately, add 10ml of chloroform, and ultrasonicate twice, each time for 15 minutes, Filtrate, combine the filtrates, evaporate to dryness, add chloroform to the residue to make up to 1ml, and obtain the test solution. Weigh 5 mg of 3-O-(2,3-dimethylbutyryl)-13-O-dodecanoyl ingenol reference substance, weigh it accurately, add methanol to dissolve and set the volume to the mark to prepare 1.00mg / ml reference substance solution. Precisely draw 20 μl of each of the above-mentioned reference substance solution and the test solution, inject them into a high-performance liquid chromatograph respectively, use octadecylsilane bonded silica gel as a filler, and use methanol-water as the mobile phase, and the gradient elution is (time / methanol percentage): 0 to 5 minutes / 70% to 85%, 5 to 55 minutes / 85% to 95%, the...
Embodiment 2
[0024] (1) Get 10 batches of Gansui decoction pieces purchased from Shanghai, Anhui, Hebei and the laboratory, and measure 3-O-(2,3-dimethylbutyryl) therein according to the method of Example 1(1) -13-O-lauroyl ingenol content. As a result, the content of 3-O-(2,3-dimethylbutyryl)-13-O-dodecanoylingenol in 10 batches of Gansui decoction pieces ranged from 0.27 to 0.92 mg / g.
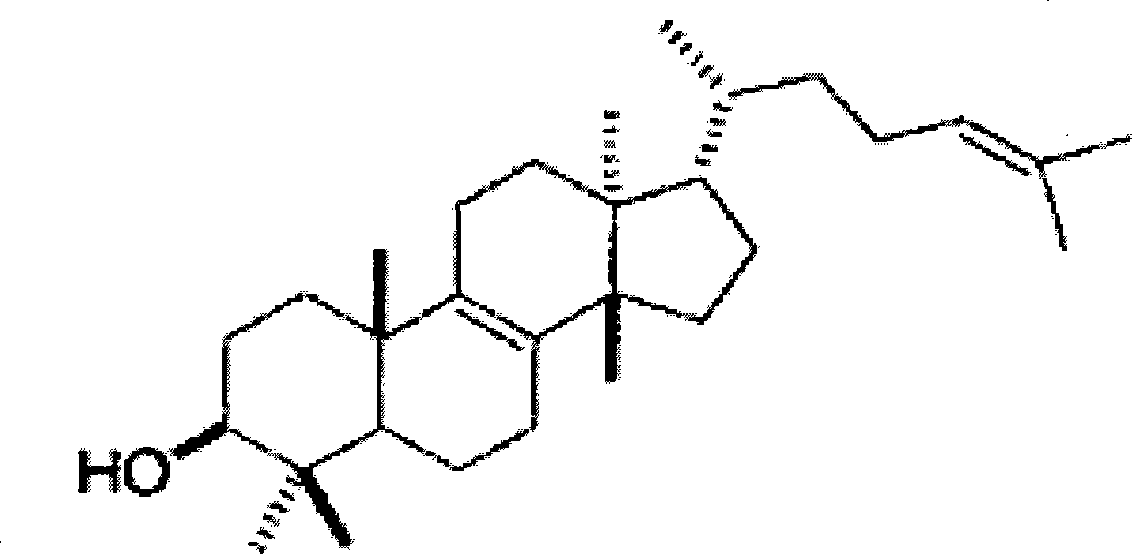
[0025] (2) Take 10 batches of Gansui decoction pieces purchased from Shanghai, Anhui, Hebei and the laboratory, and determine the content of euphoradienol in it according to the method of Example 1 (2). As a result, the content of euphora in 10 batches of Gansui decoction pieces ranged from 1.49 to 4.5 mg / g.
[0026] It can be judged from this example that all 10 batches of Gansui decoction pieces purchased from Shanghai, Anhui, Hebei and the laboratory are qualified products.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com