Methods of detecting autoantibodies for diagnosing and characterizing disorders
A technology for autoantibodies and disorders, applied in the fields of disease diagnosis, chemical method analysis, measurement devices, etc., can solve the problem of not being able to truly detect the early stage of the target disorder
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0125] Reactive Antibodies Linked to Cancer
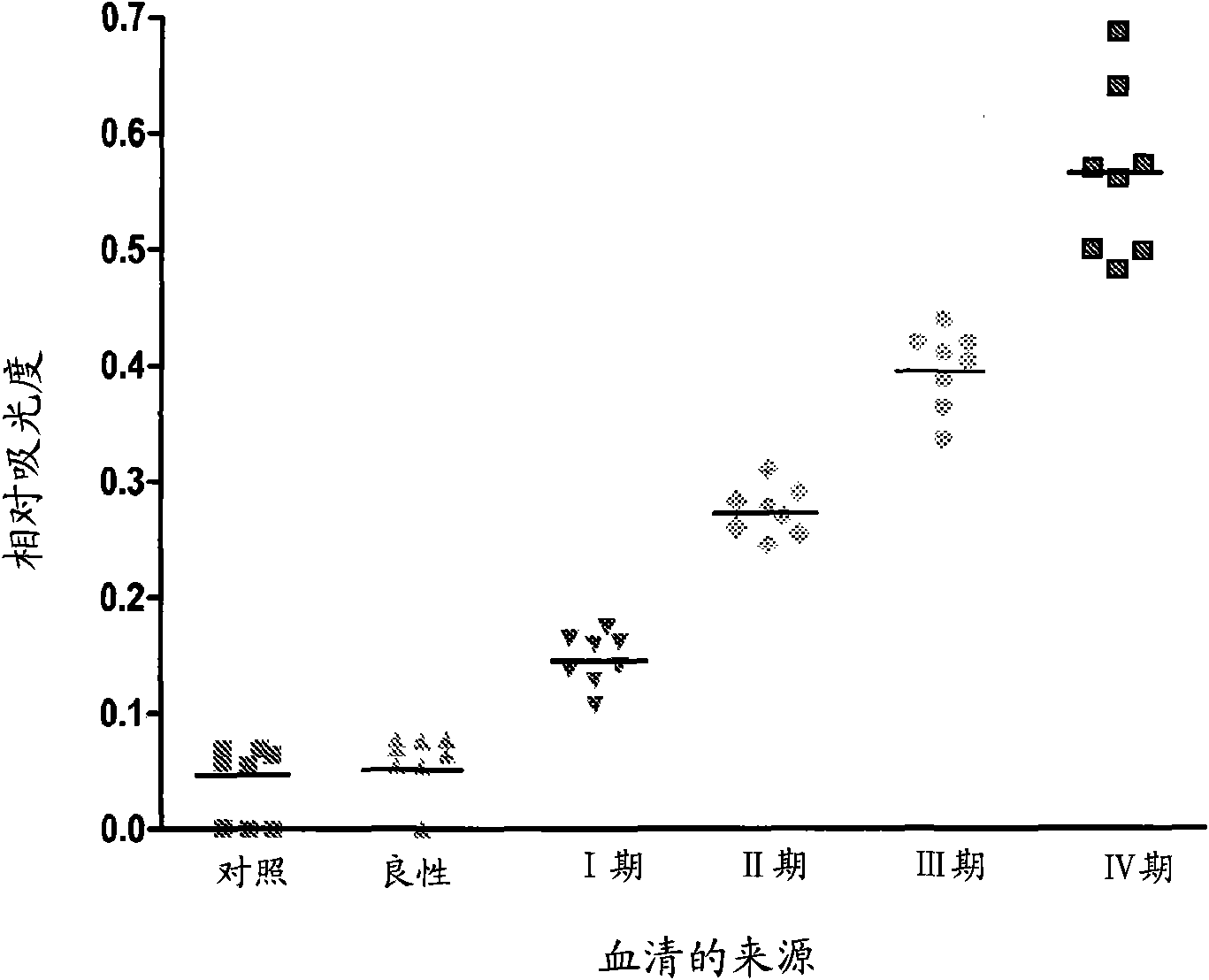
[0126] Autoreactive antibodies have been shown to be prevalent in all cancer patients tested. The presence of tumor-reactive autoantibodies was analyzed in ovarian cancer patients (n=28) and tumor-free age-matched female volunteers (n=32). The presence of IgG in ovarian cancer patient sera and their reactivity with ovarian tumor-derived cellular antigens were quantified by ELISA.
[0127] Tumor-derived cell antigens from UL-1 ovarian tumor cells in logarithmic growth phase were diluted 1:25 into coupling buffer (pH 8.3) consisting of 100 mM sodium carbonate and 0.5 M NaCl. Aliquots were added to wells of a 96-well Immulon4 microtiter plate and incubated overnight at 37°C. The plates were blocked with 5% non-fat dry milk and then incubated overnight at 4°C with 200 μl of serum diluted 1 / 200 from ovarian cancer patients and age-matched normal controls. The plate was washed and incubated with peroxidase-conjugated human IgG antibod...
Embodiment 2
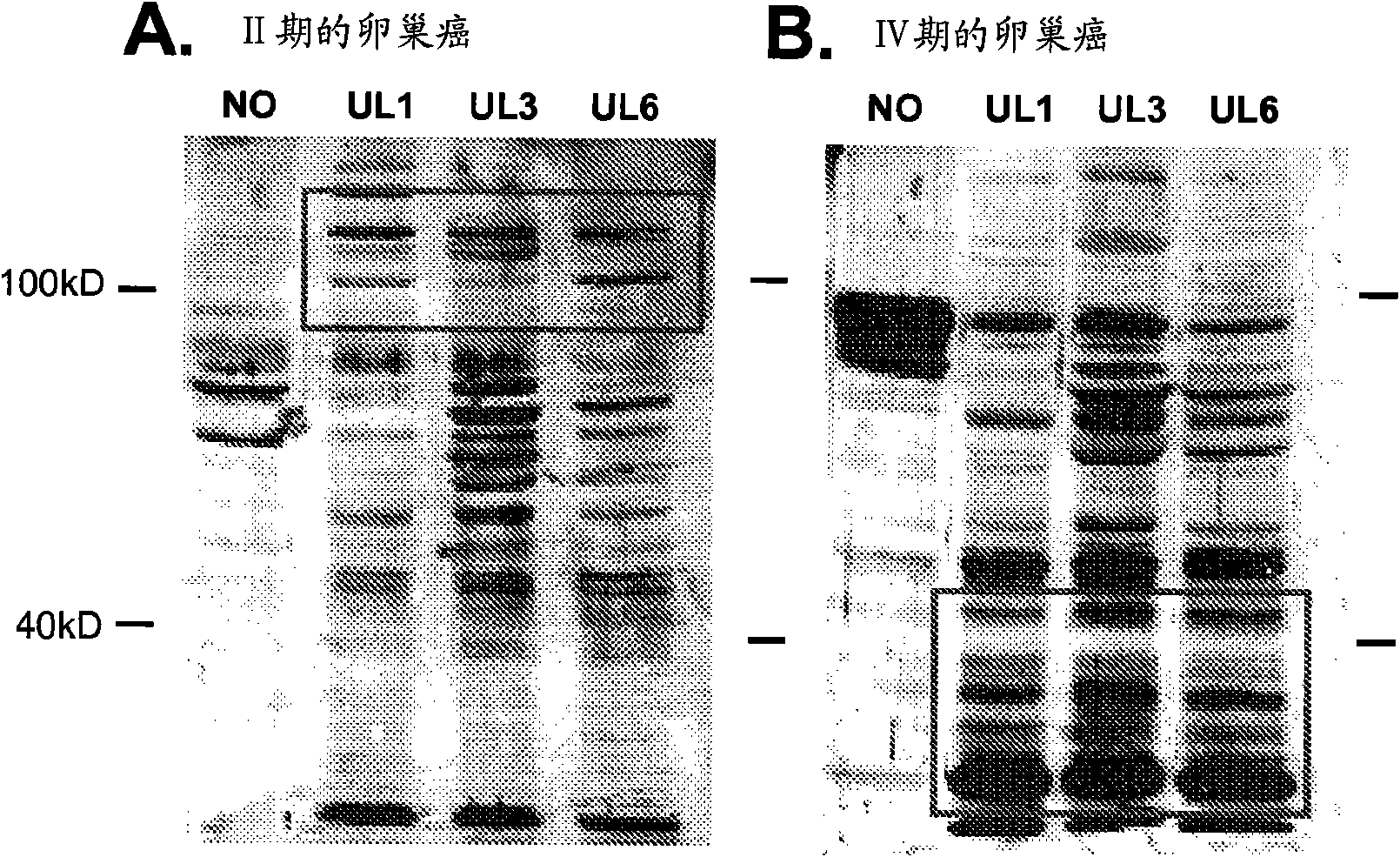
[0131] Specific antigen target recognition by tumor-reactive IgG
[0132] Since the reactivity of autoantibodies from ovarian cancer patient sera to ovarian tumor antigens can exhibit quantitative differences based on the stage of the disease, qualitative differences were also investigated using Western blot and densitometry. Using cellular protein antigens from three ovarian tumor cell lines (UL-1, UL-3, and UL-6) in logarithmic growth phase, the activity of components reactive with the humoral immune response of ovarian cancer patients was assessed by Western blotting. exist( figure 2 , representative blot). Western blotting was performed using patient serum (1:100 dilution) as the primary antibody source. The binding of the patient-derived antibody to the tumor cell-derived antigen was visualized using ECL, first using a peroxidase-conjugated human IgG antibody. Immunoreactivity was quantified by densitometry. The antigens recognized and differences in the strength of ...
Embodiment 3
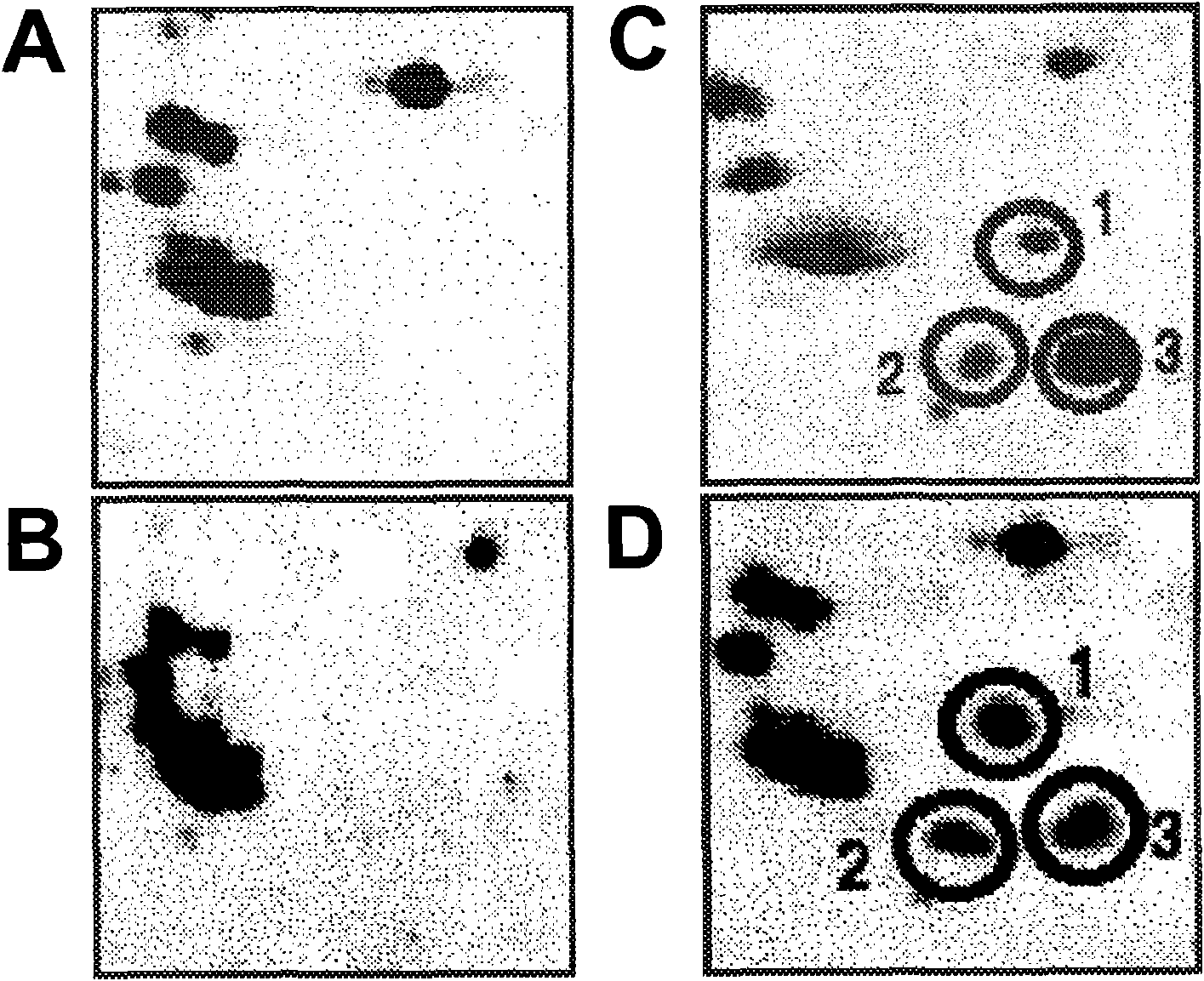
[0138] Marker Identification for Treatment Response
[0139] Since differences in reactivity patterns occurred in patients with advanced disease, the correlation between this difference and chemoresistance was examined. Separation of cellular components by 2-dimensional electrophoresis followed by western blotting allowed some of these differences to be assessed. Identification of a cluster of 3 points associated with cisplatin resistance was identified using sera from patients who did not initially respond to cisplatin therapy and patients who presented an initial response and remained disease-free for >12 months ( image 3 ). Utilization of these cellular antigens as part of the diagnostic arrays disclosed herein allows early identification of resistant tumors.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com