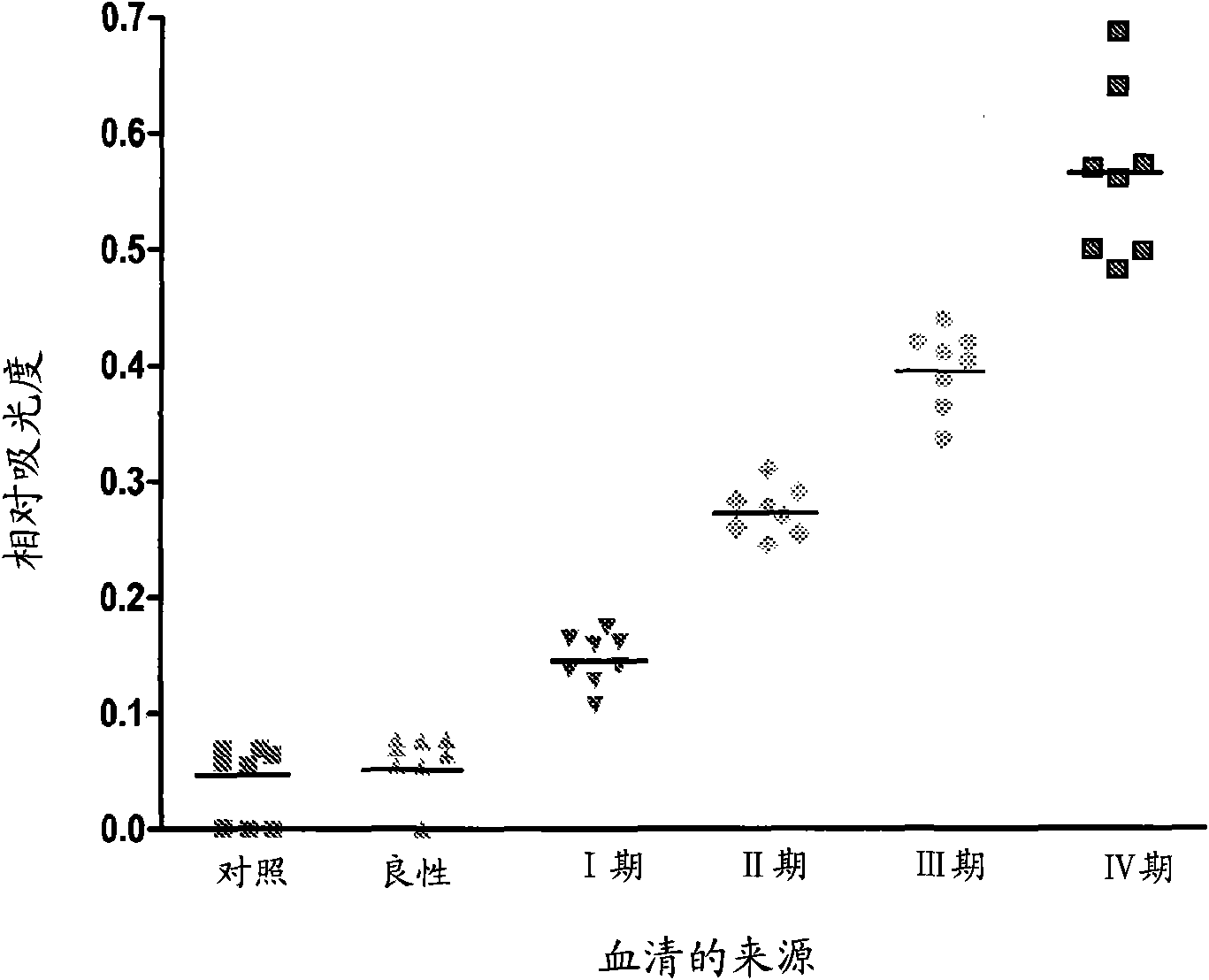
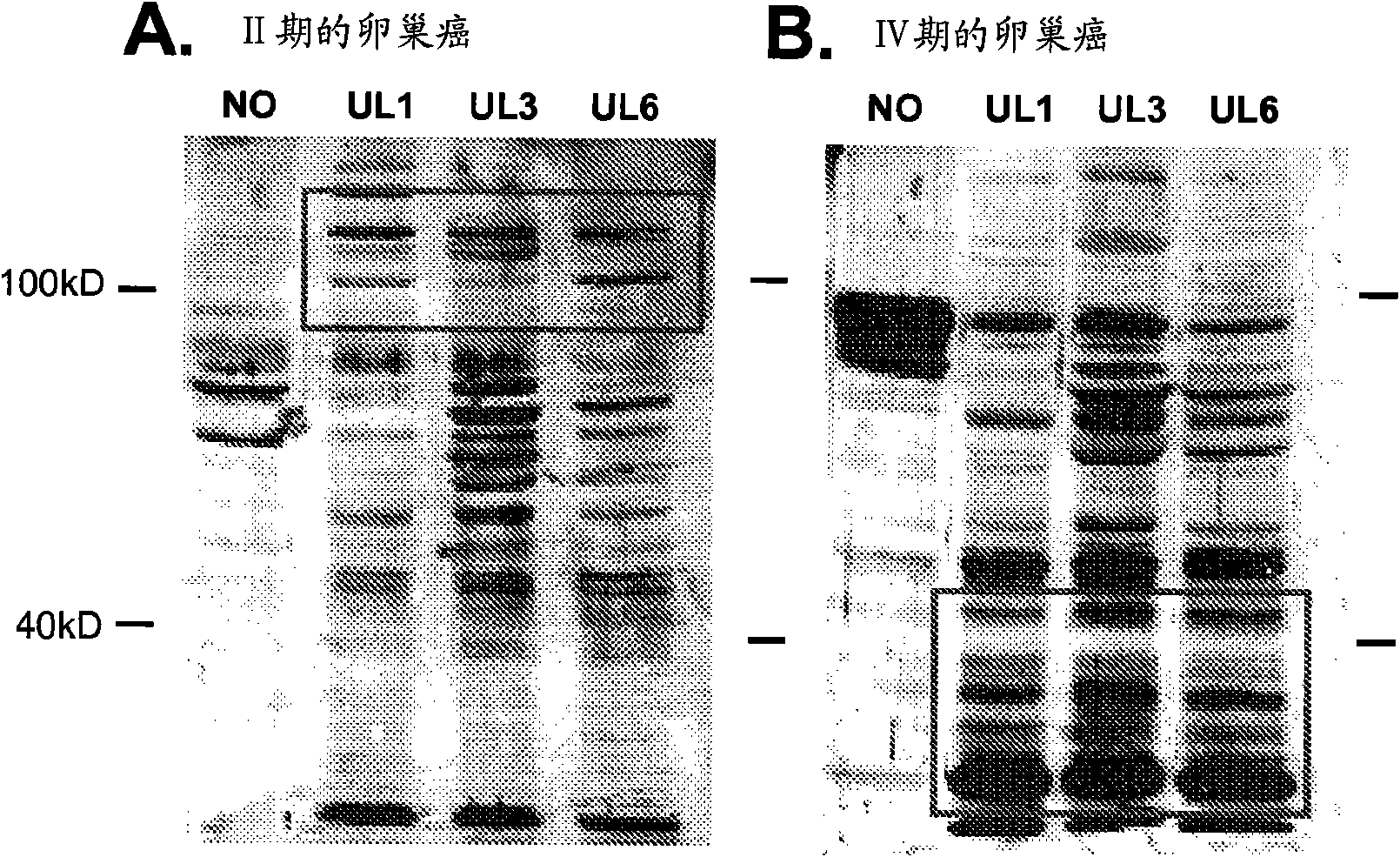
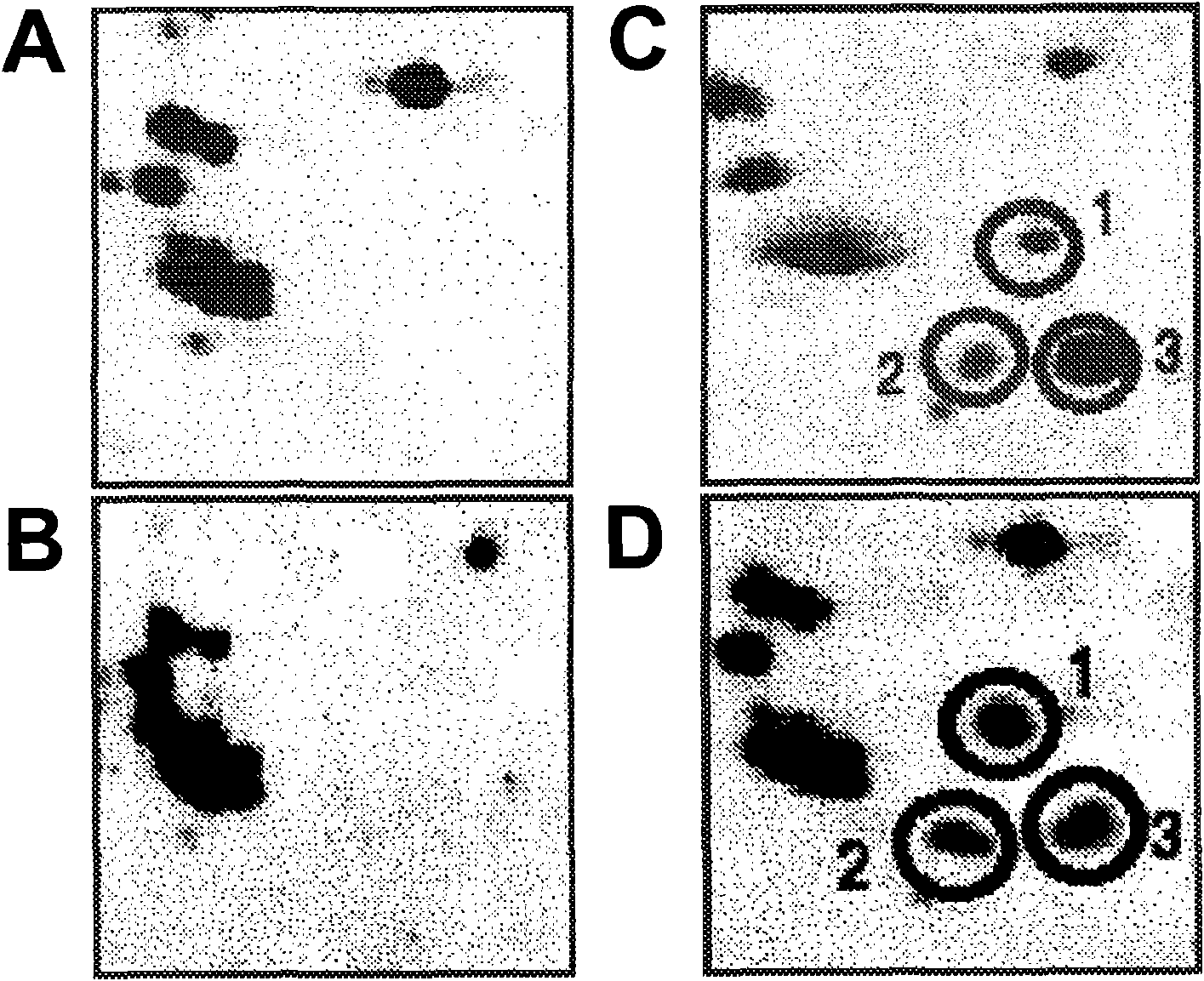
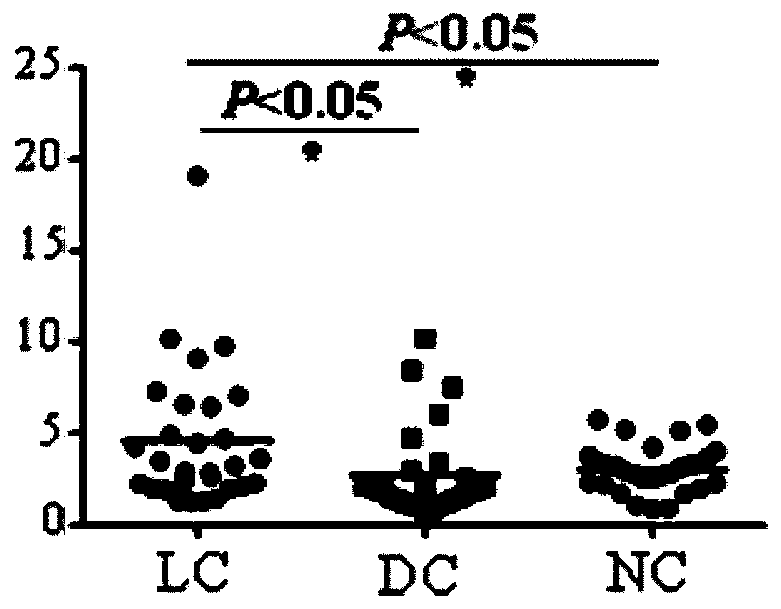
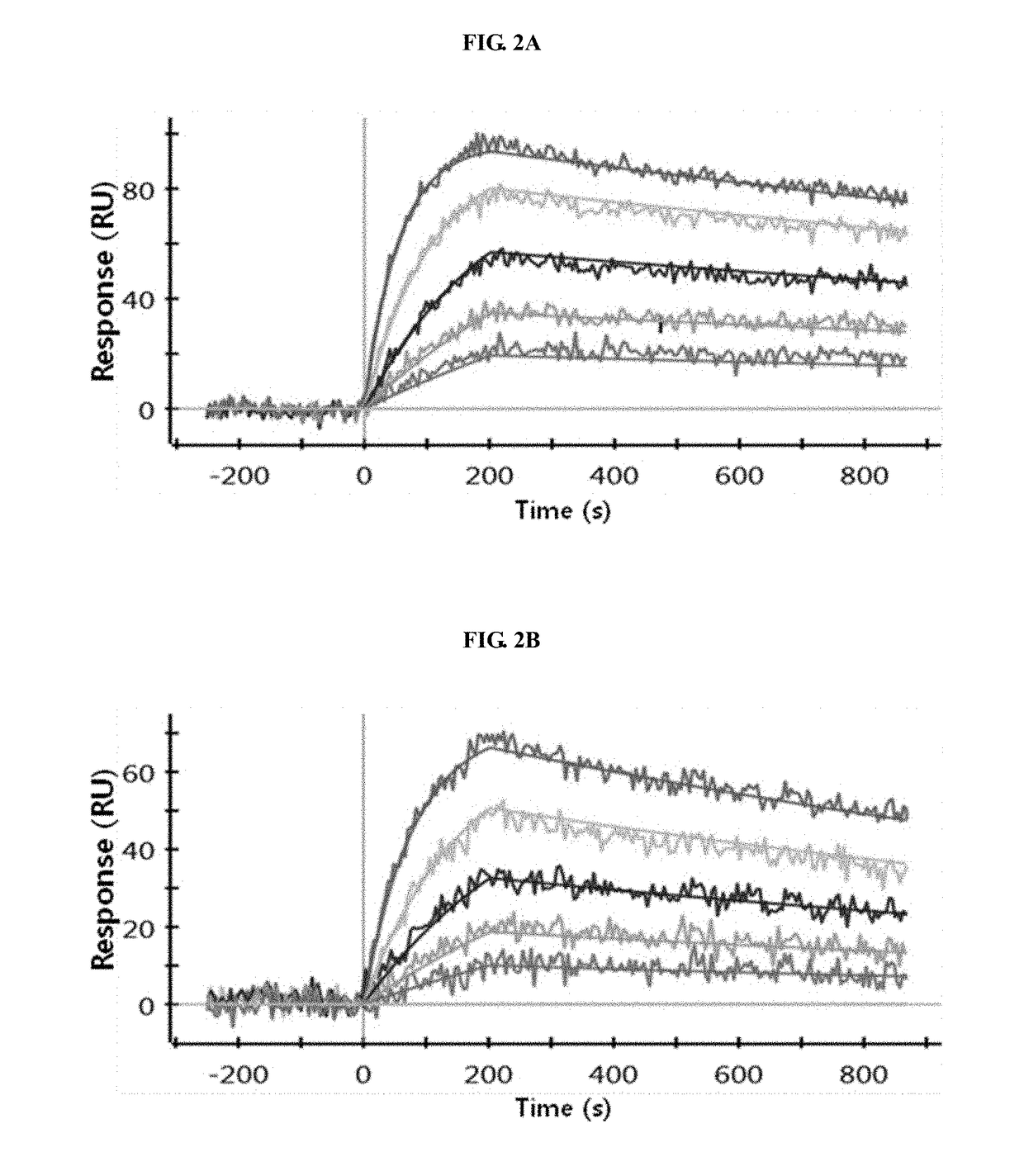
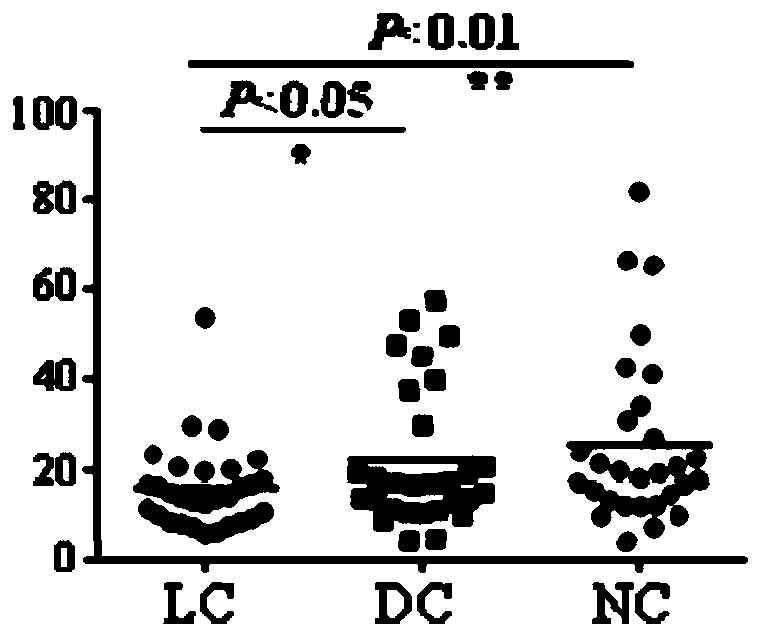
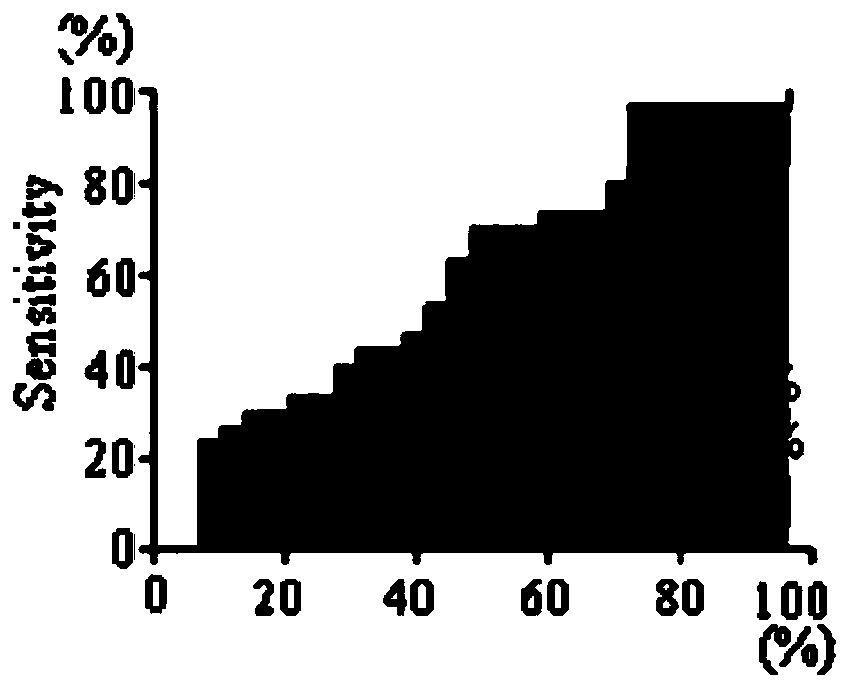
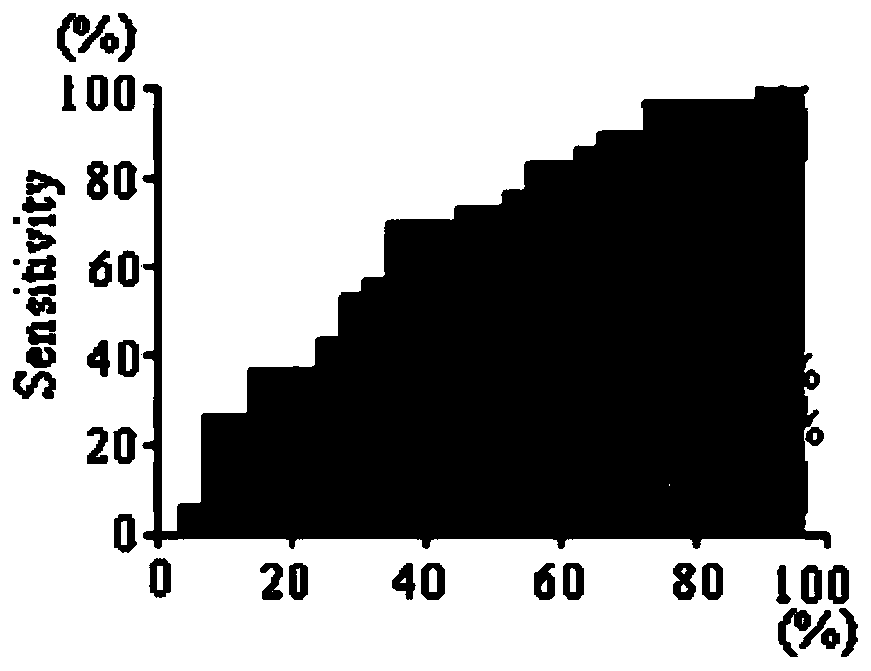
Patents
Literature
114 results about "Autoantibody level" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Phosphorylcholine Conjugates and Corresponding Antibodies
ActiveUS20070286868A1Increased and decreased riskSignificant positive effectImmunoglobulins against animals/humansAntibody ingredientsPhosphorylcholineIgm antibody
IgG and IgM autoantibody levels against phosphorylcholine in subjects with hypertension (diastolic pressure>95 mmHg) were determined at baseline in order to determine the importance of antibodies for the development of atherosclerosis. The results show that increases in intima-media thickness (IMT) at a follow-up four years after baseline were significantly less prevalent in subjects having high IgM autoantibodies to phosphorylcholine. The presence or absence of IgM autoantibodies against phosphorylcholine is thus related to an increased or decreased risk of developing ischemic cardiovascular diseases. A method to determine IgM antibodies toward phosphorylcholine is proposed in this invention to identify subjects at risk of developing ischemic cardiovascular diseases. Animal experiments show that medium to high levels of IgM antibodies can be detected in plasma after active immunization with a keyhole limpet hemocyanin (KLH)-phosphorylcholine conjugate. A pharmaceutical composition comprising a phosphorylcholine conjugate (active immunization) or a monoclonal antibody with specificity to a phosphorylcholine conjugate (passive immunization) is proposed and the use of these compositions as active or passive immunogens in the treatment or prevention of atherosclerosis.
Owner:ATHERA BIOTECH
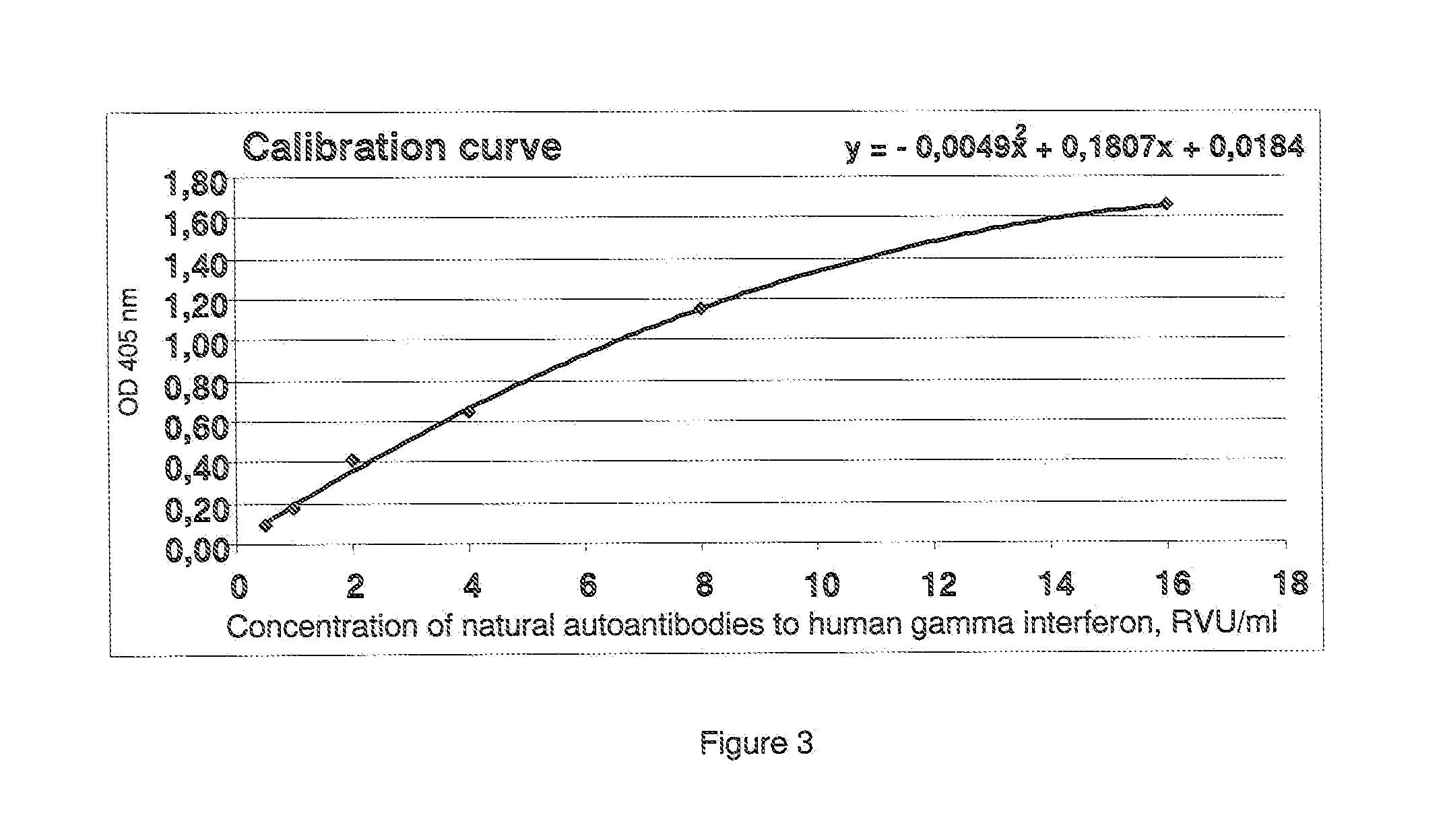
Method of determination of autoantibody level by means of enzyme immunoassay
InactiveUS20130189707A1High sensitivityReduce sensitivityBiological testingBiotin-streptavidin complexSolid phases
The method for quantitative determination of the level of natural autoantibodies in human biological fluids, when as a solid phase of physical sorption is used the solid phase of physical sorption, coated with streptavidin, and the solid phase of physical sorption is treated with preliminary biotinylated antigen and blocking agent for closing the sites of nonspecific binding at the solid phase of physical sorption, for which purpose are used proteins, biotinylated according to standard procedure. As the conjugate-containing solution are used enzyme-labeled monoclonal and polyclonal antibodies, which react with one or all isotypes of human immunoglobulins. In addition, the tested biological fluid is preliminary diluted in a buffer, containing proteins which are used for closing the sites of nonspecific binding at solid phase of physical sorption, and also substances protecting natural autoantibodies from destruction during heat treatment, and subjected to heat treatment. For each tested specimen of biological fluid, a control solid phase of physical sorption is used, and the number of natural autoantibodies is determined with the use of a calibration curve which is plotted using monoclonal or polyclonal antibodies to antigen.
Owner:SERGEEVA SVETLANA ALEXANDROVNA +3
Application of ERP27 autoantibody detection reagent to preparation of lung cancer screening kit
ActiveCN110108879AEfficient screeningReduce harmBiological material analysisBiological testingSerum igeAutoantibody production
The invention relates to the field of in vitro diagnostic reagents, in particular to application of an ERP27 autoantibody detecting reagent to preparation of a lung cancer screening kit. It is found for the first time that the autoantibody level of ERP27 proteins in serum of lung cancer patients is significantly higher than that of healthy patients. The reagent for detecting the autoantibody of the ERP27 proteins is used for preparing the lung cancer screening kit, and lung cancers can be effectively screened.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Methods of detecting autoantibodies for diagnosing and characterizing disorders
InactiveCN101657719ADisease diagnosisChemical methods analysisAutoantibody productionAutoantibody level
Methods for detecting and / or quantitating levels of autoantibodies in subjects are provided. Methods for diagnosing and / or characterizing a disorder associated with autoantibody production are furtherprovided. In some embodiments, the disorder diagnosed and / or characterized can be a cancer or an infertility disorder.
Owner:UNIV OF LOUISVILLE RES FOUND INC
Application of FAM172A autoantibody detection reagent in preparing lung cancer screening kit
ActiveCN110108877AEfficient screeningReduce harmBiological material analysisBiological testingAutoantibody levelMedicine
The invention relates to the field of in vitro diagnostic reagents, and particularly relates to an application of an FAM172A autoantibody detection reagent in preparing a lung cancer screening kit. The scheme of the invention finds for the first time that the autoantibody level of FAM172A protein in the serum of lung cancer patients is significantly higher than that of healthy patients. The schemeof the invention uses a reagent for detecting an FAM172A protein autoantibody to prepare the lung cancer screening kit, and can realize the effective screening of lung cancer.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Antibody binding to fcrn for treating autoimmune diseases
ActiveUS20170210805A1High affinityStrong specificityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsSerum igeAutoimmune disease
The present disclosure relates to an isolated anti-FcRn antibody, which is an antibody binding to FcRn (stands for neonatal Fc receptor, also called FcRP, FcRB or Brambell receptor) that is a receptor with a high affinity for IgG or a fragment thereof, a method of preparing thereof, a composition for treating autoimmune disease, which comprises the antibody, and a method of treating and diagnosing autoimmune diseases using the antibody. The FcRn-specific antibody according to the present disclosure binds to FcRn non-competitively with IgG to reduce serum pathogenic auto-antibody levels, and thus can be used for the treatment of autoimmune diseases.
Owner:HANALL PHARMA CO LTD
Application of SARS2 autoantibody detection reagent in preparation of lung cancer screening kit
The invention relates to the field of the in-vitro diagnosis reagent, and specifically relates to an application of SARS2 autoantibody detection reagent in preparation of a lung cancer screening kit.The condition that the autoantibody level of the SARS2 protein in the lung cancer patient serum is remarkably higher than that of the health patient is firstly discovered in the invention, the reagentfor detecting the autoantibody of the SARS2 protein is used for preparing the lung cancer screening kit, thereby realizing effective screening of the lung cancer.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +1
Use of PDE9A autoantibody detection reagent in preparing lung cancer screening kit
The invention relates to the field of in vitro diagnostic reagents, in particular to the use of a PDE9A autoantibody detection reagent in preparing a lung cancer screening kit. The use of a PDE9A autoantibody detection reagent in preparing a lung cancer screening kit finds that the level of autoantibodies of PDE9A protein in serum of lung cancer patients is significantly higher than that of healthy patients for the first time. The use of a PDE9A autoantibody detection reagent in preparing a lung cancer screening kit uses the reagent for detecting the PDE9A protein autoantibody to prepare a lung cancer screening kit, and can effectively screen the lung cancer.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +1
Construction method of systemic lupus erythematosus animal model and application thereof
ActiveCN107693542AHigh induced molding rateRapid onsetMammal material medical ingredientsAnimal husbandryDiseaseDendritic cell
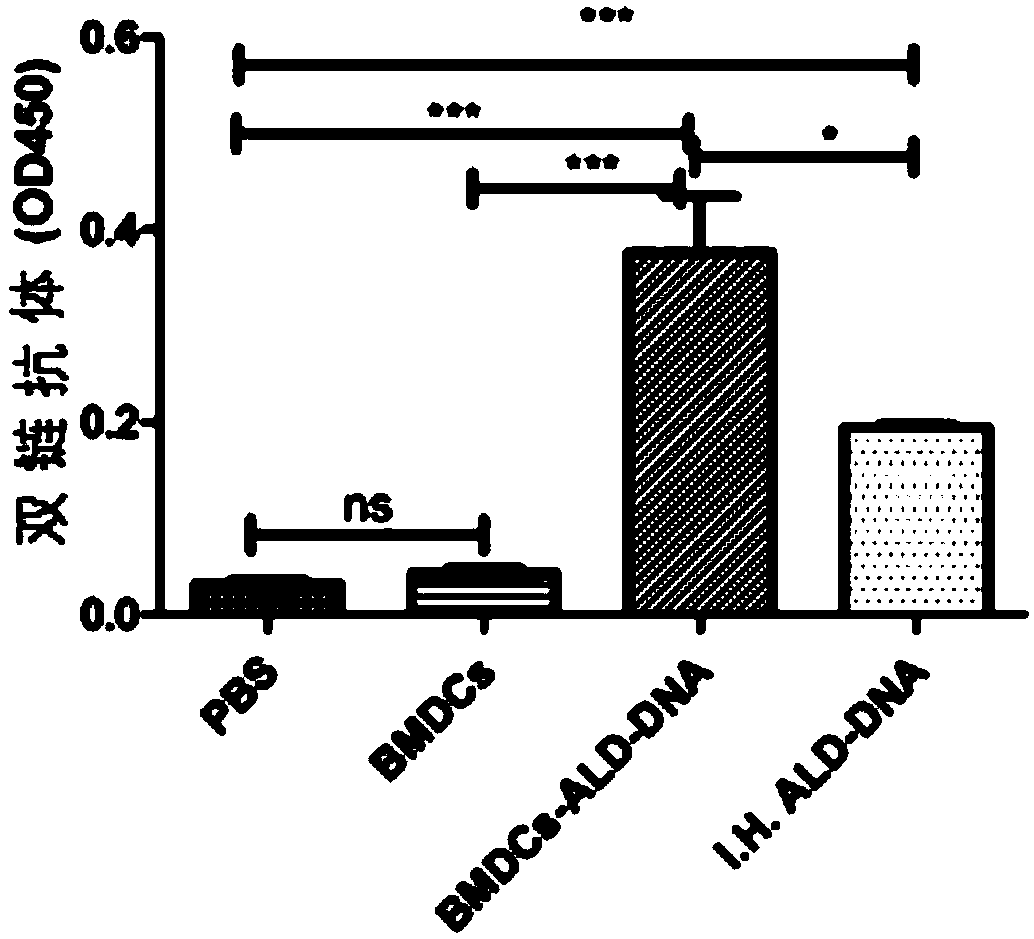
The invention discloses a method for constructing an animal model of systemic lupus erythematosus. According to the method, after dendritic cells of mice are coated in vitro with lymphocyte DNA in apoptosis state for 12 hours, the dendritic cells are tail-vein injected into mice body circulation to induce systemic lupus erythematosus in mice. Systemic lupus erythematosus disease in mice is evaluated by detecting the increase of autoantibody level in mice, hyperplasia and swelling of mice lymphoid tissues, and later changes in renal glomerular inflammation traits in mice. This method can help induce mice systemic lupus erythematosus within 2 weeks, and the induction model success rate reaches up to 100%. The method of the invention is a rapid, concise, efficient, immune cell and immunogen clear construction method of the systemic lupus erythematosus animal model, and can facilitate the exploration of the mechanism of systemic lupus erythematosus disease and the search for new and effective treatment methods.
Owner:THE THIRD AFFILIATED HOSPITAL OF SUN YAT SEN UNIV
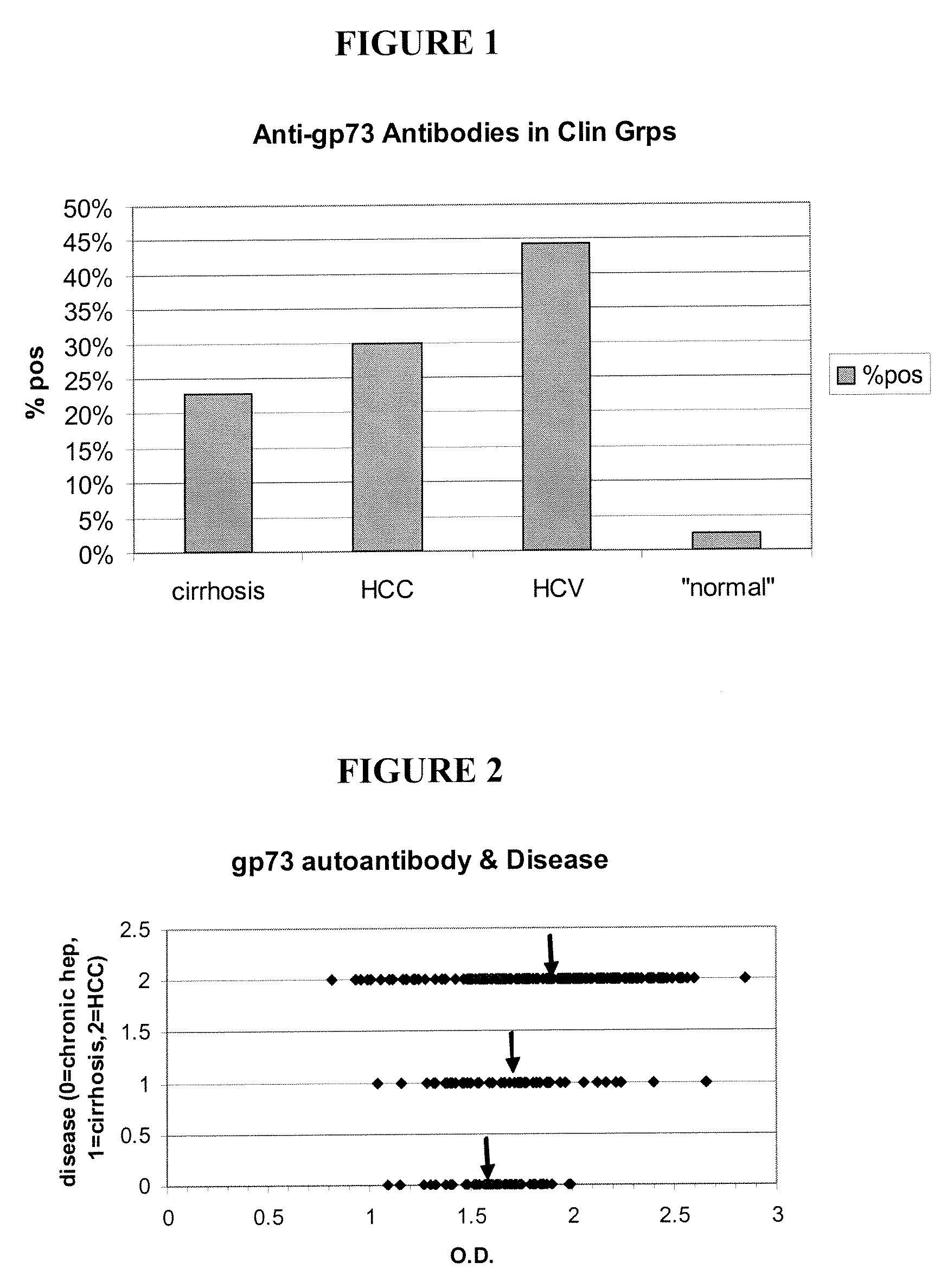
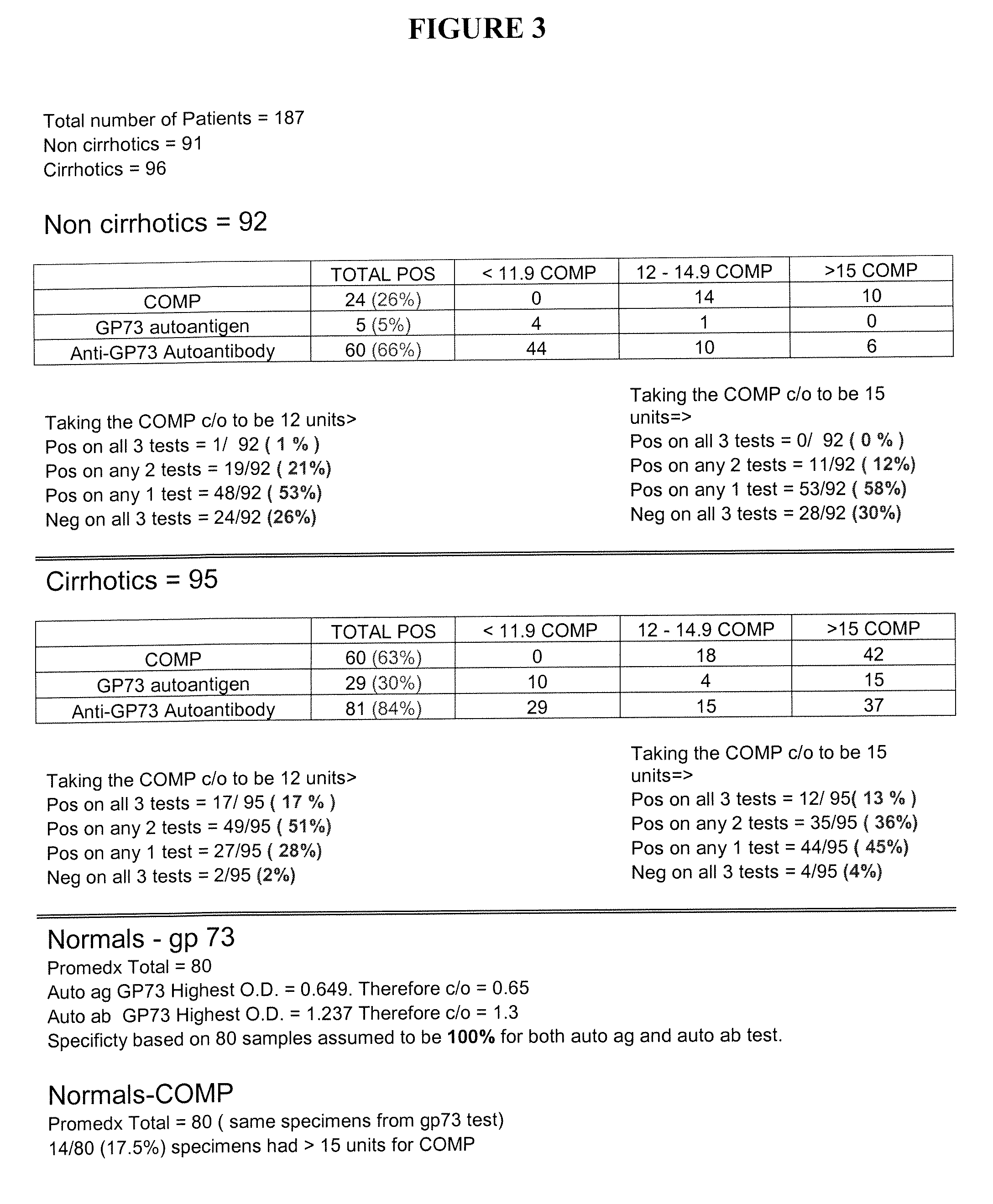
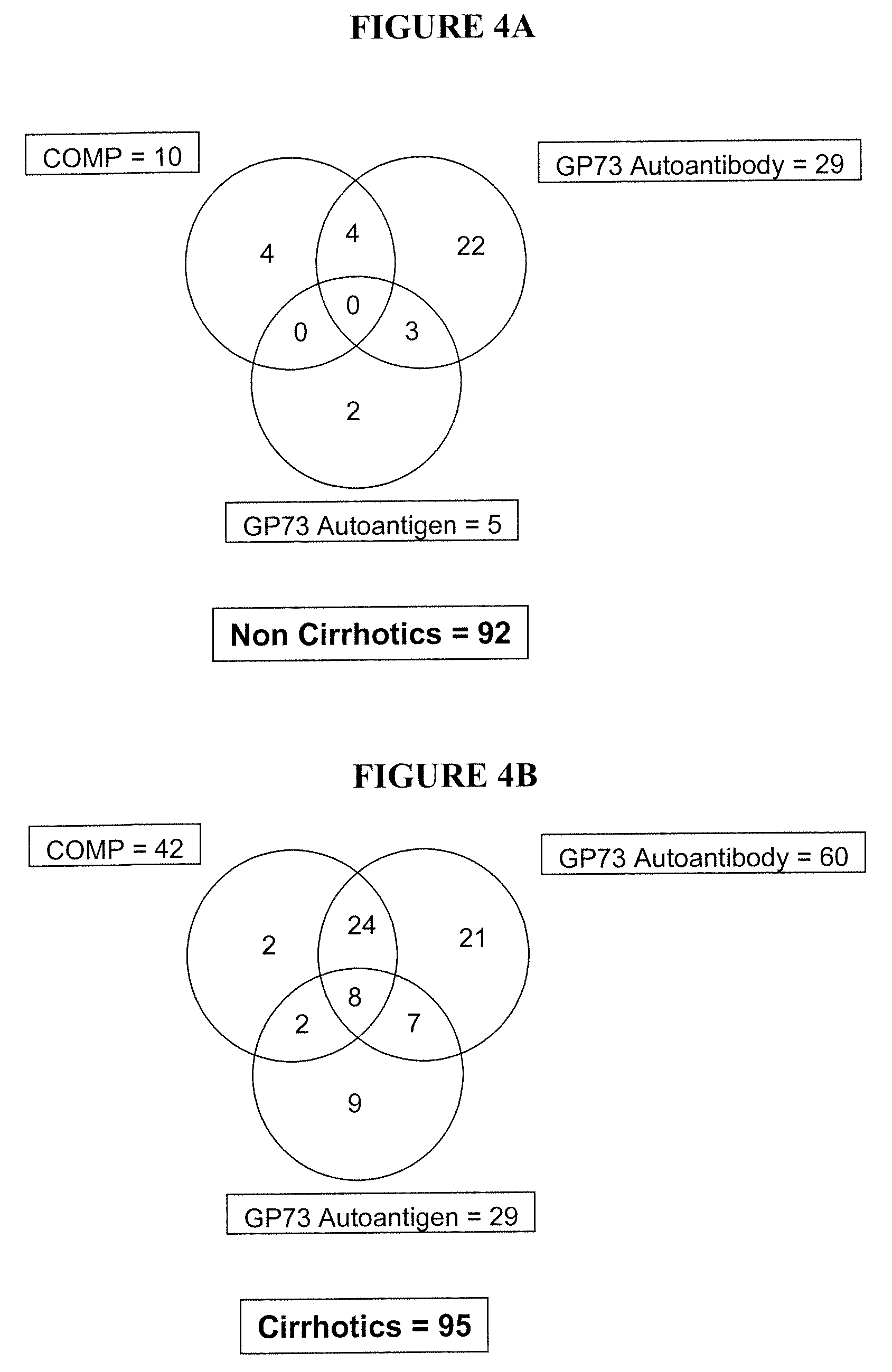
Methods and assays for detecting GP73-specific autoantibodies
The present invention provides a method for detecting autoantibodies in a subject which reacts with a GP73 antigen. Increased levels of GP73-specific autoantibodies in a sample from the subject which bind to GP73 antigen are indicative of liver disease in the subject.
Owner:INOVA DIAGNOSTICS INC
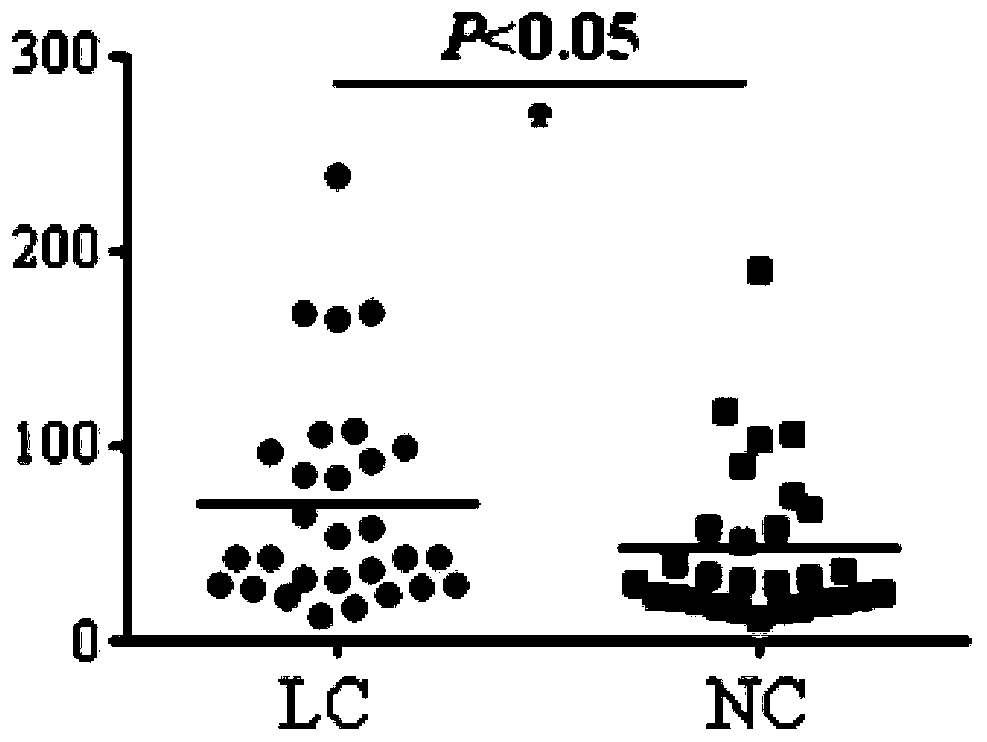
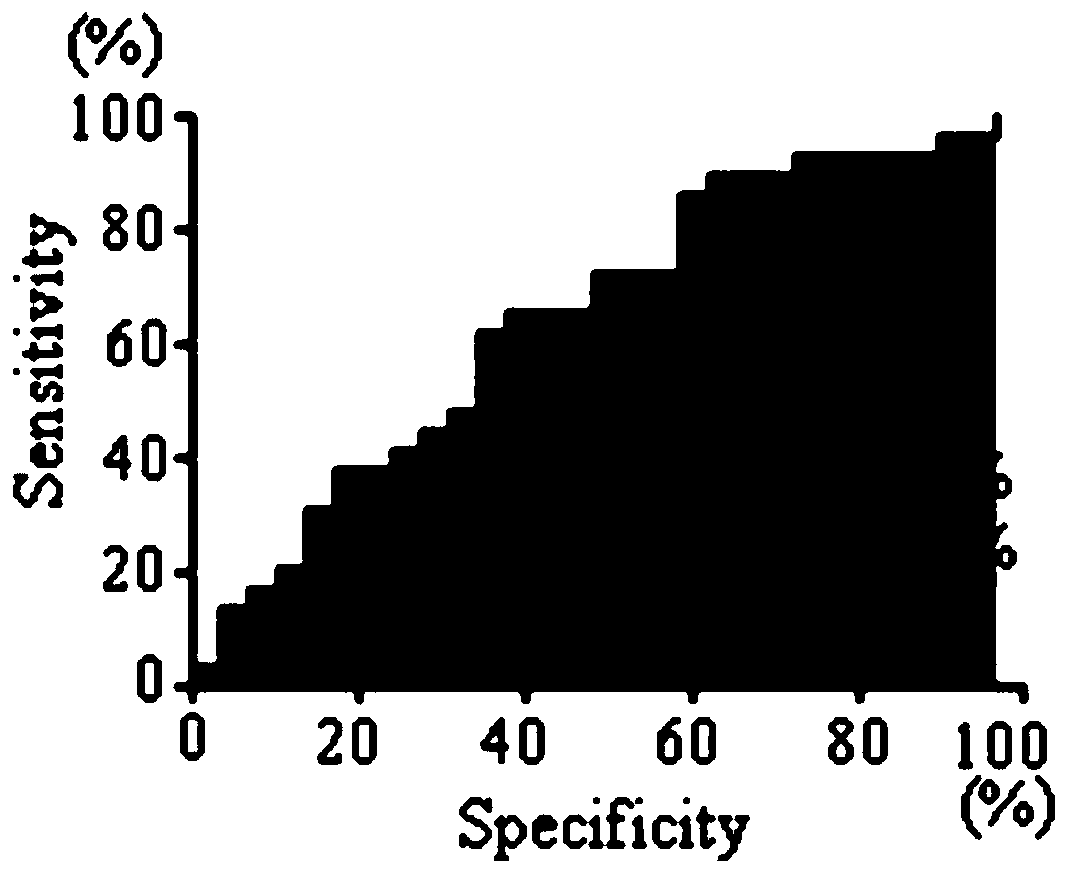
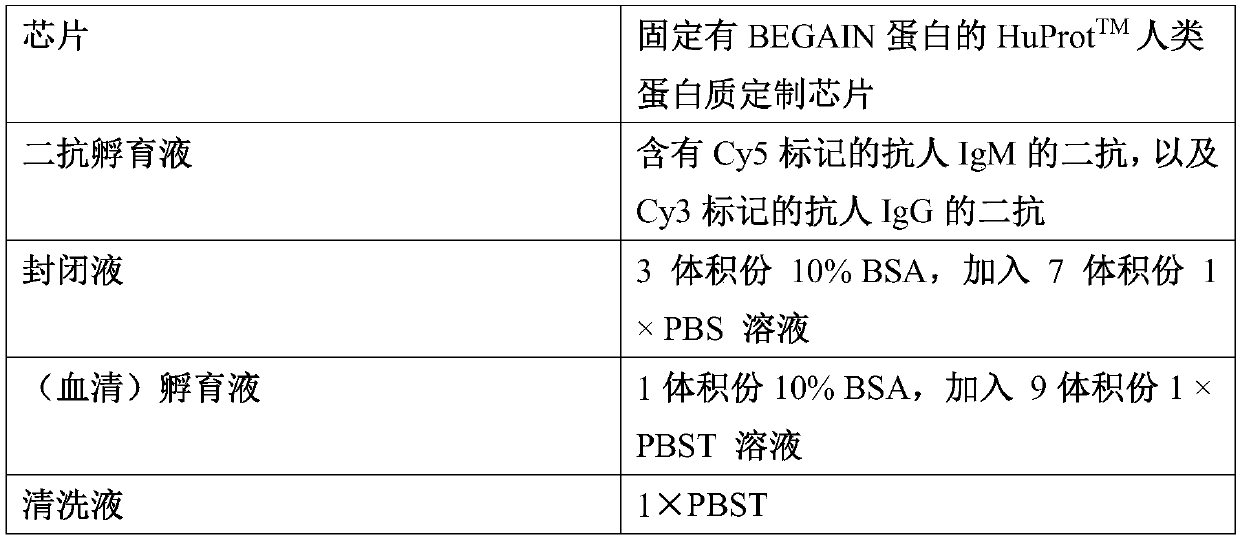
Application of BEGAIN own antibody detection agent in preparation of lung cancer screening kit
The invention relates to the field of the in vitro diagnostic reagent, and specifically relates to an application of the BEGAIN own antibody detection reagent in the preparation of a lung cancer screening kit. The detection agent disclosed by the invention firstly discovers that the own antibody level of the BEGAIN protein in the lung cancer patient serum is obviously higher than the health patient. The reagent for detecting the BEGAIN protein own antibody is used for preparing the lung cancer screening kit, and the effective screening of the lung cancer can be realized.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +1
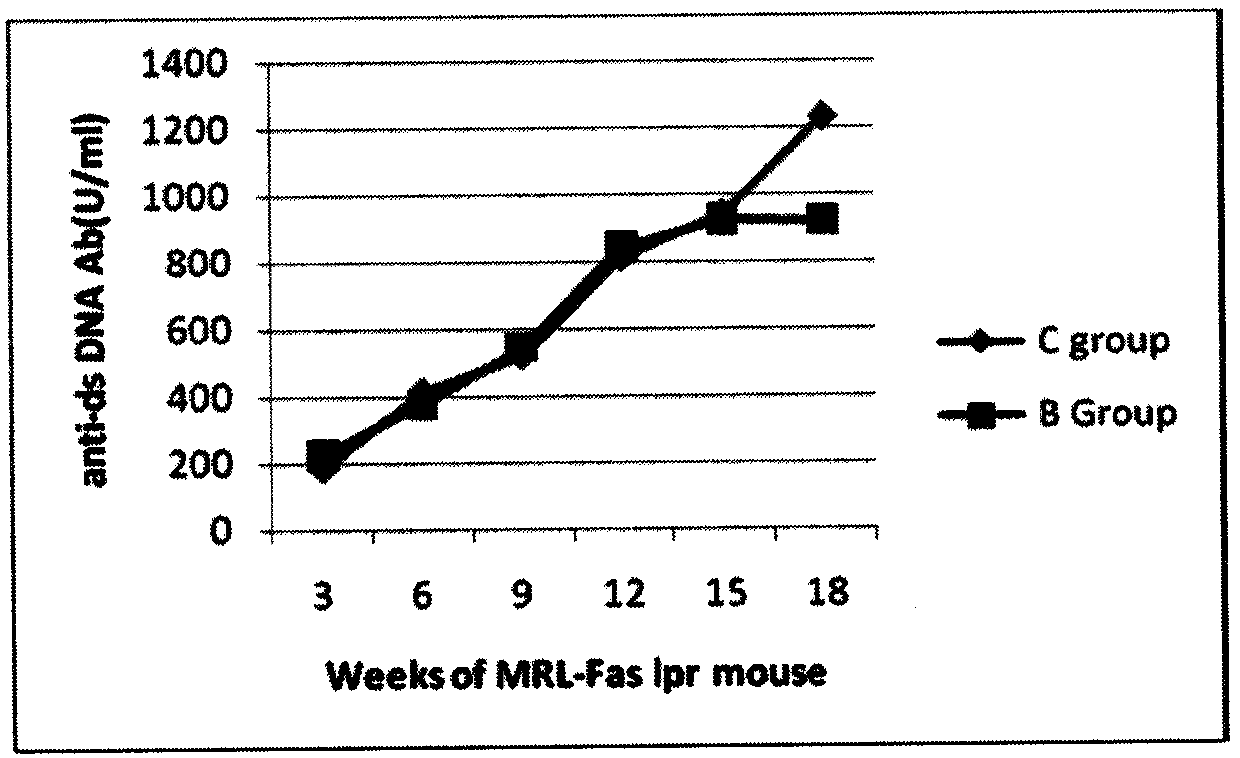
Application of 2-methoxyestradiol in treatment of systemic lupus erythematosus
InactiveCN104188974AReduce nephritis symptomsLower autoantibody levelsOrganic active ingredientsImmunological disordersDiseaseEstradiolum
The invention discloses application of 2-methoxyestradiol (2ME2) in treatment of systemic lupus erythematosus. 2ME2 can be used as a therapeutic medicine for systemic lupus erythematosus or a component contained in the therapeutic medicines. The systemic lupus erythematosus includes cutaneous lupus, lupus nephritis, lupus encephalopathy and other systemic or / and organic lupus diseases. 2ME2 is given in a tail vein injection manner with a dosage of 0.75 mu M / kg each time, or is given in an intragastric manner with a dosage of 100mu M / kg each time. 2ME2 is used for treating B6.MRL-Fas(lpr) mice. When used for treating lupus erythematosus model mice (MRL-Fas(lpr) mice), 2ME2 has the beneficial effects of lowering the autoantibody level of lupus erythematosus, reducing syndromes of nephritis related to lupus, and relieving lupus syndromes.
Owner:THE THIRD AFFILIATED HOSPITAL INST OF FIELD SURGERY OF PLA ARMY MEDICAL UNIV
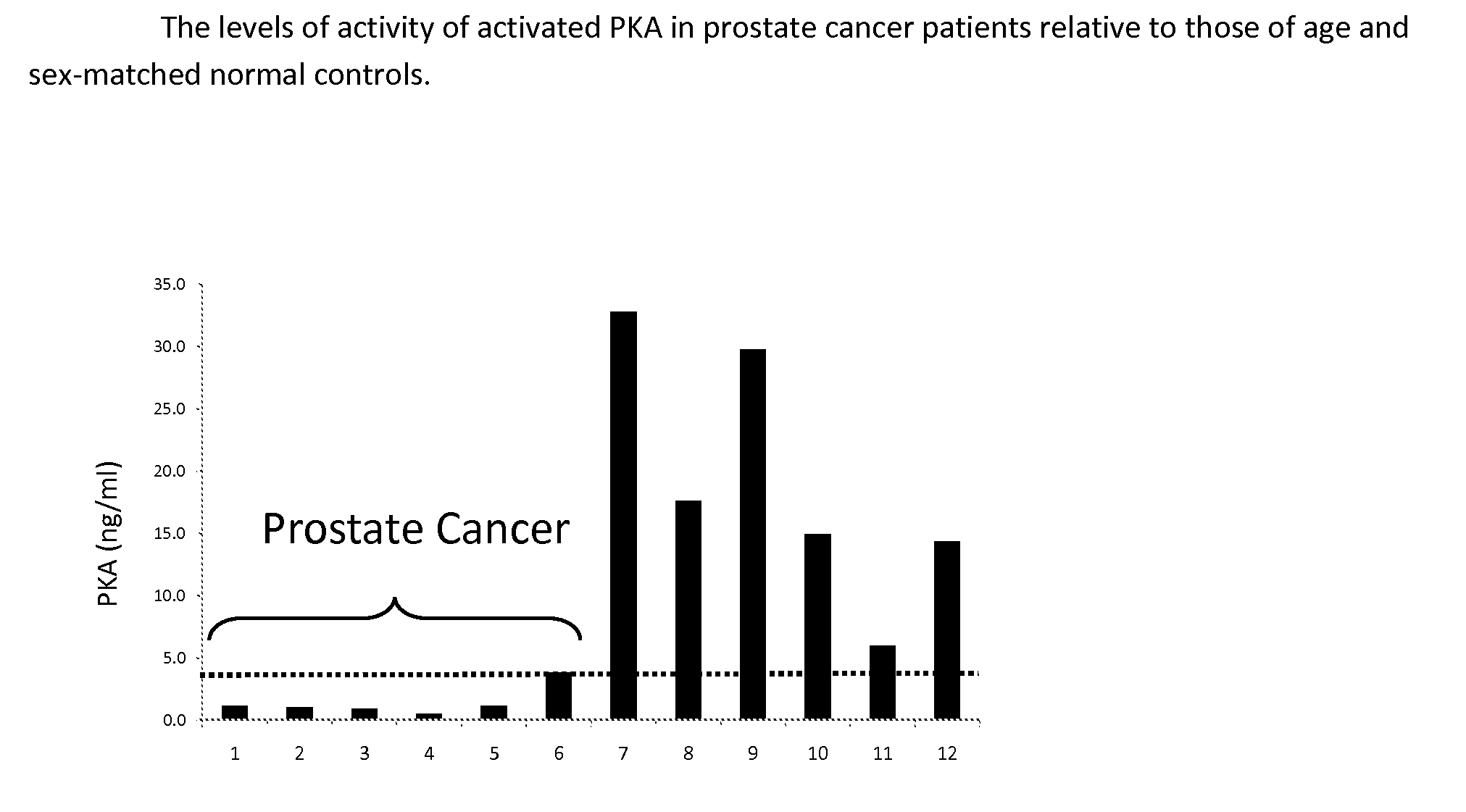
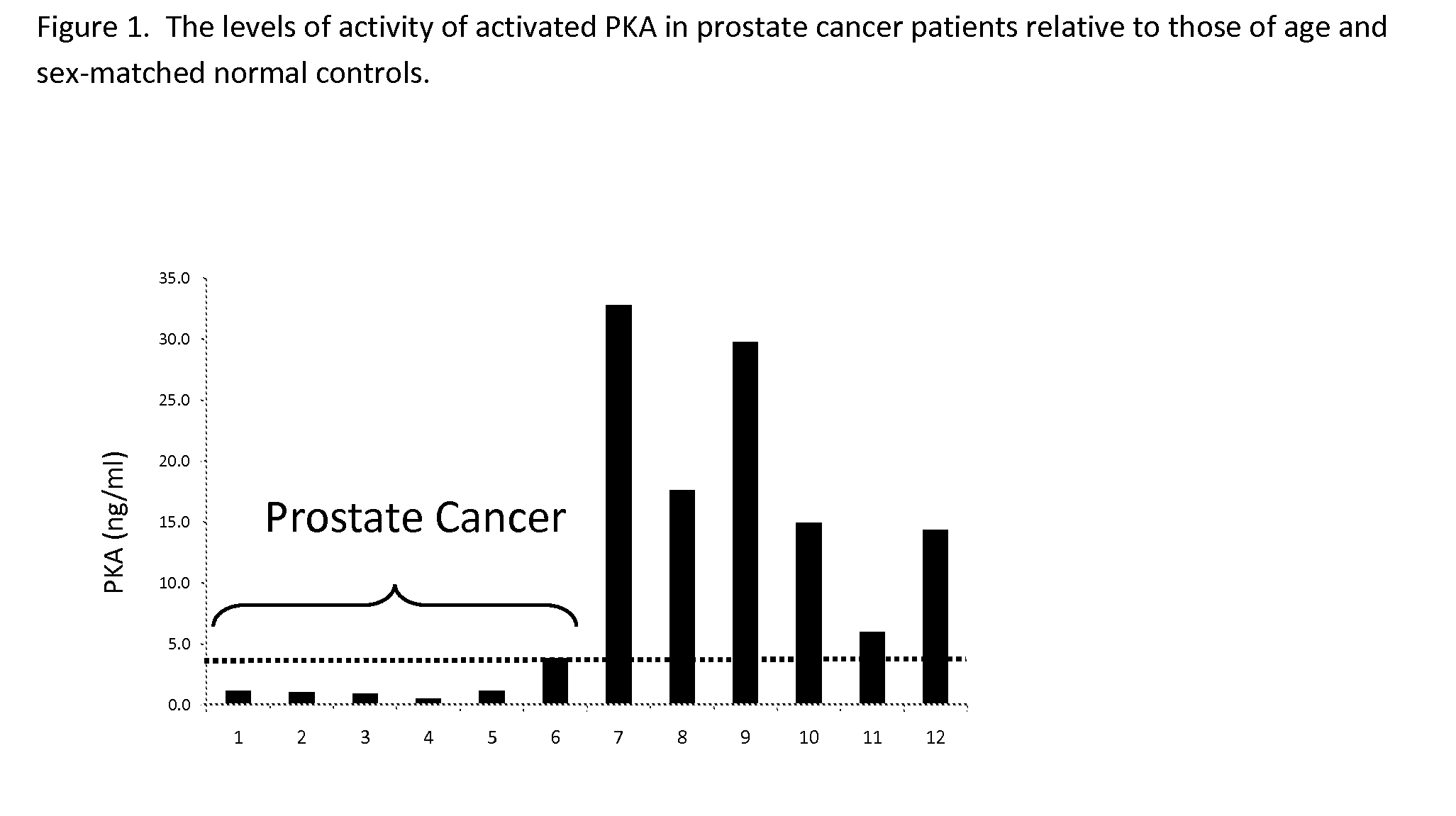
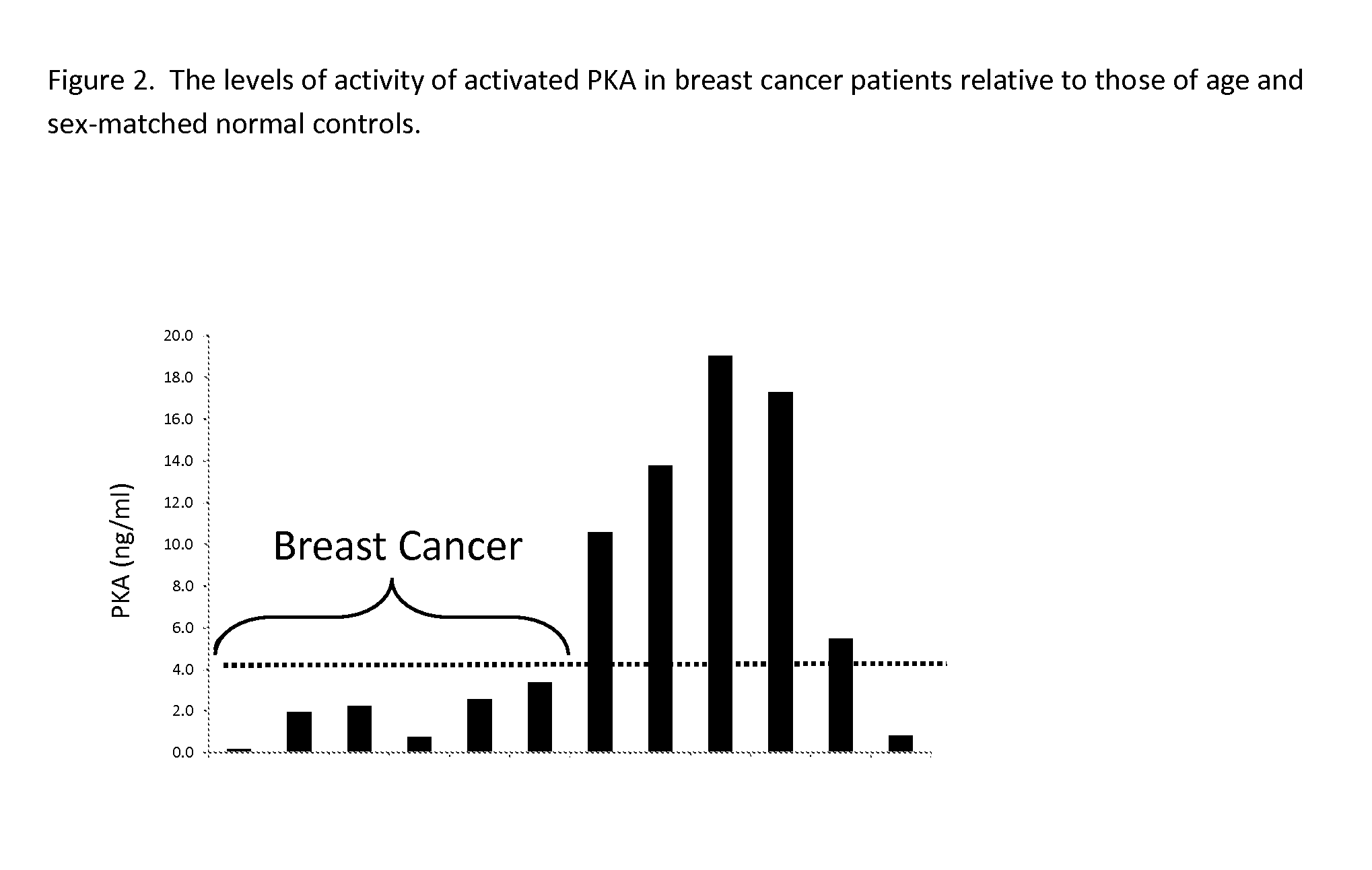
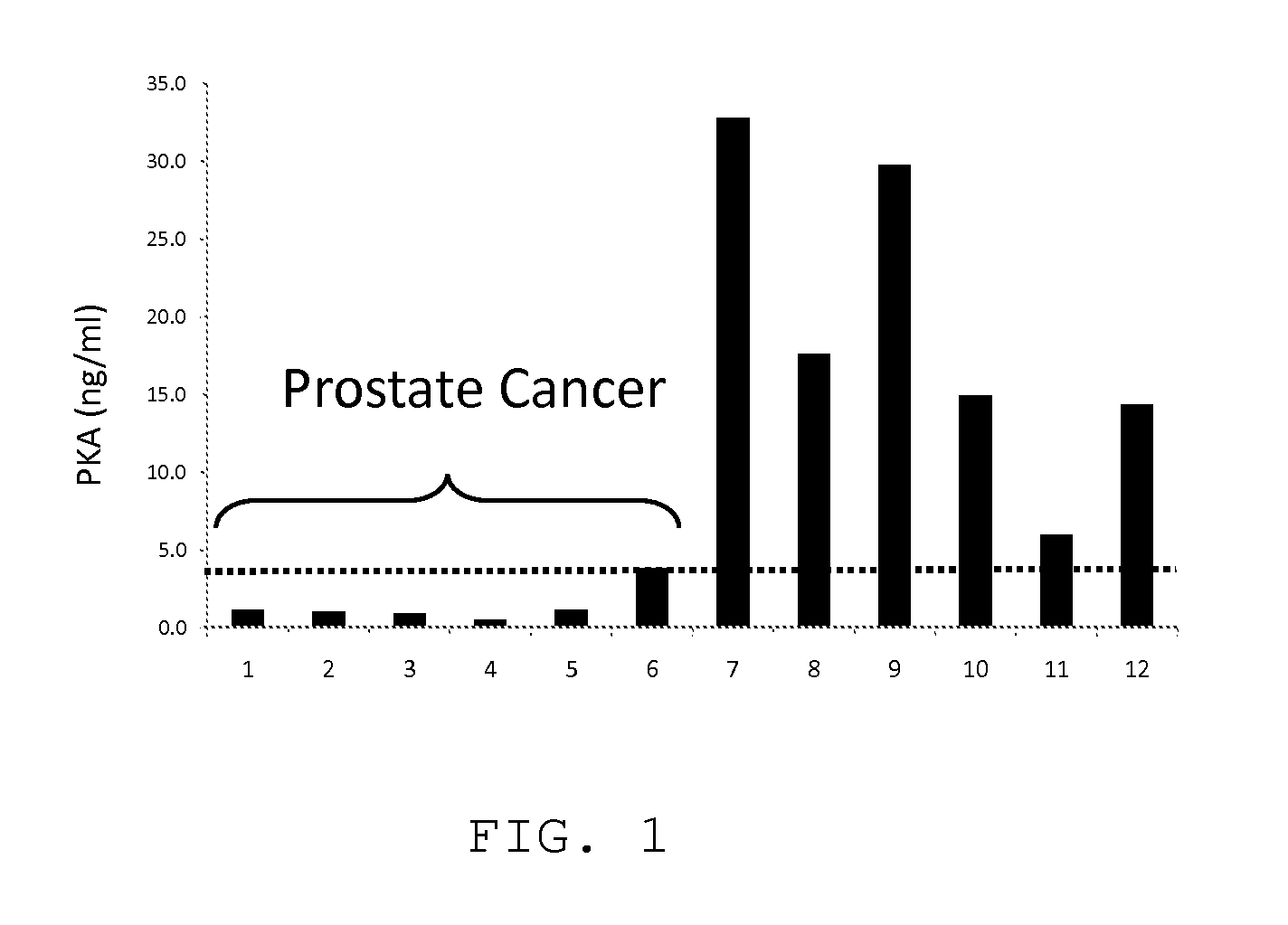
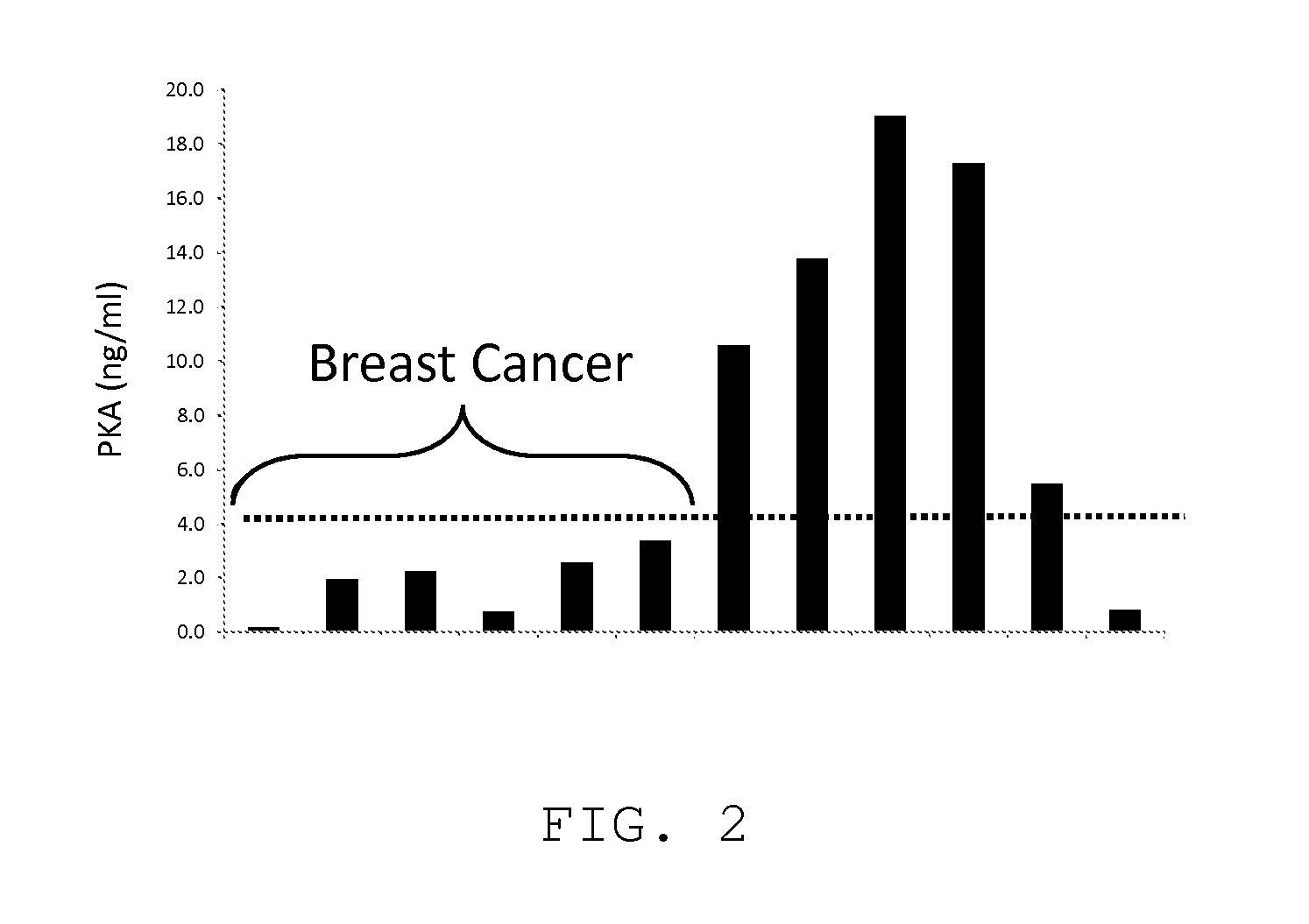
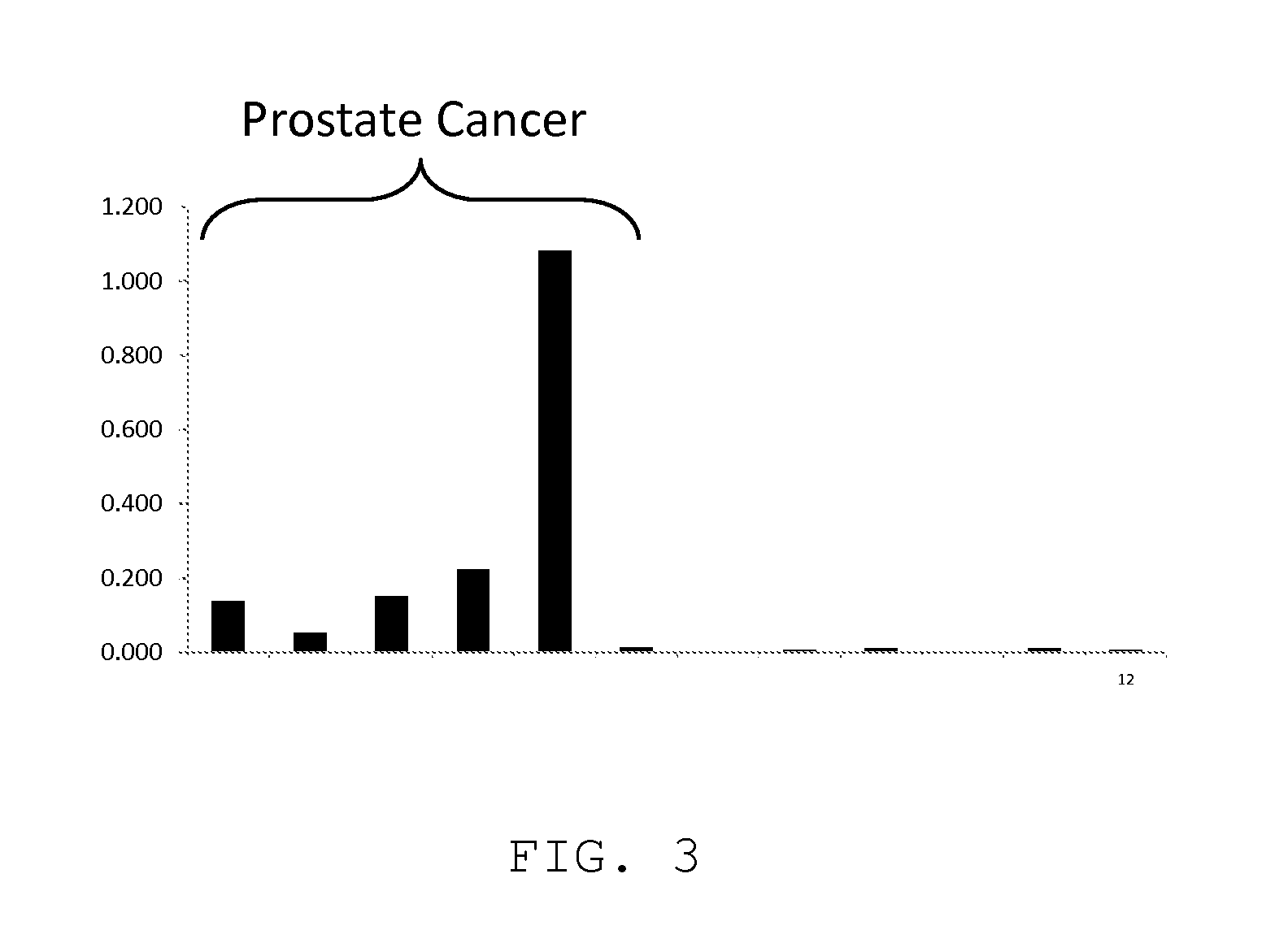
Measurement of PKA for Cancer Detection
ActiveUS20120322080A1Low levelEasy to measurePeptide/protein ingredientsMicrobiological testing/measurementAnalyteMedicine
The present invention relates to a method of detecting the presence of cancer by measuring the level of enzyme activity and autoantibodies in the blood of an individual. In particular the present invention relates to methods for measurement of activated cAMP-dependent protein kinase A (PKA) activity and antibodies to PKA, a kit for activated PKA activity measurement, and the use of the measured levels of these analytes for determining the presence of cancer.
Owner:TRAXXSSON
Application of GTF2IRD2 autoantibody detection reagent in preparation of lung cancer screening kit
The invention relates to the field of in-vitro diagnostic reagents, in particular to application of a GTF2IRD2 autoantibody detection reagent in preparation of a lung cancer screening kit. It is foundfor the first time that the autoantibody level of the GTF2IRD2 protein in serum of a lung cancer patient is significantly higher than that of a healthy patient. The reagent for detecting the GTF2IRD2protein autoantibody is used for preparing the lung cancer screening kit, and effective screening of lung cancer can be achieved.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
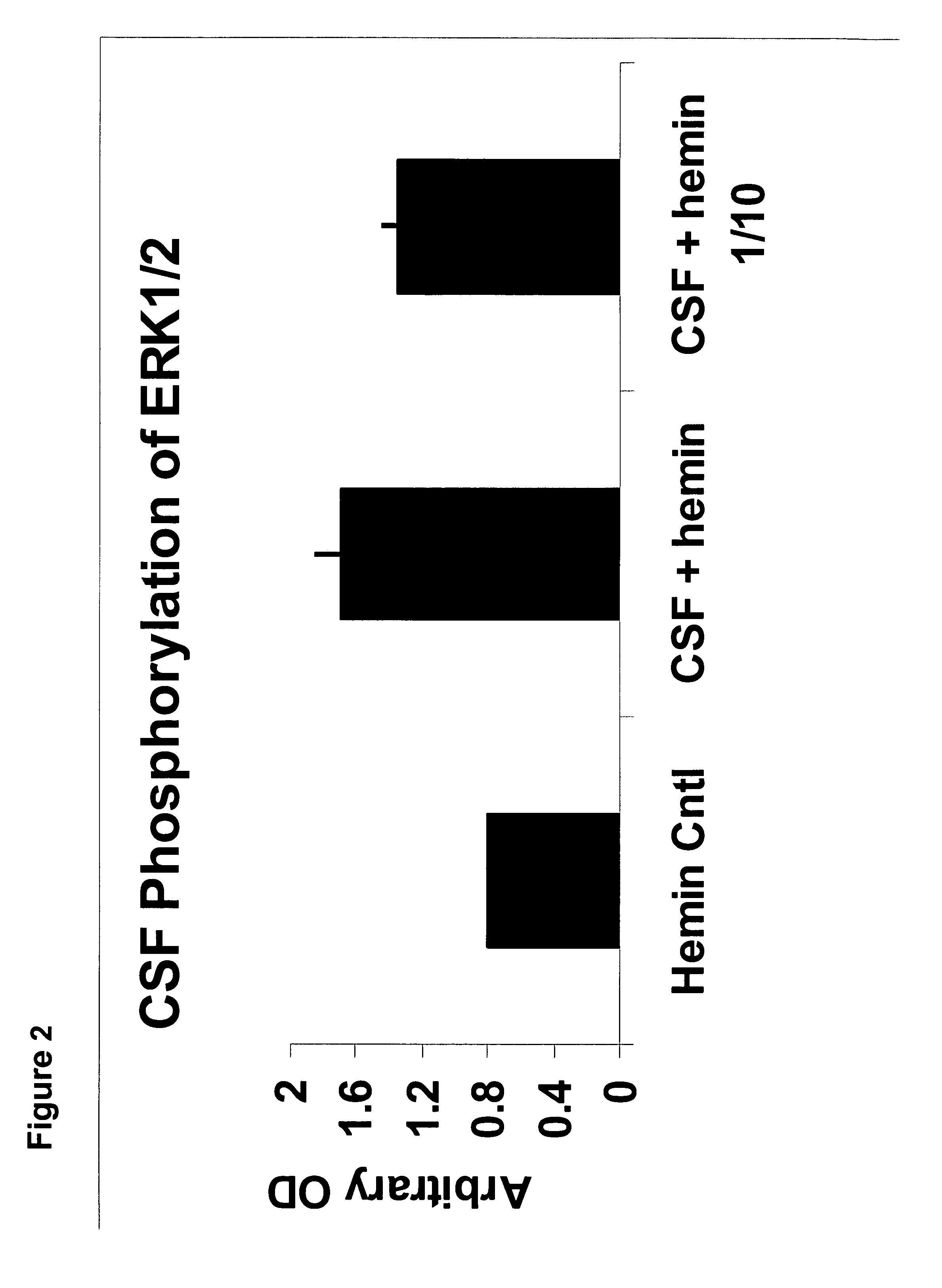
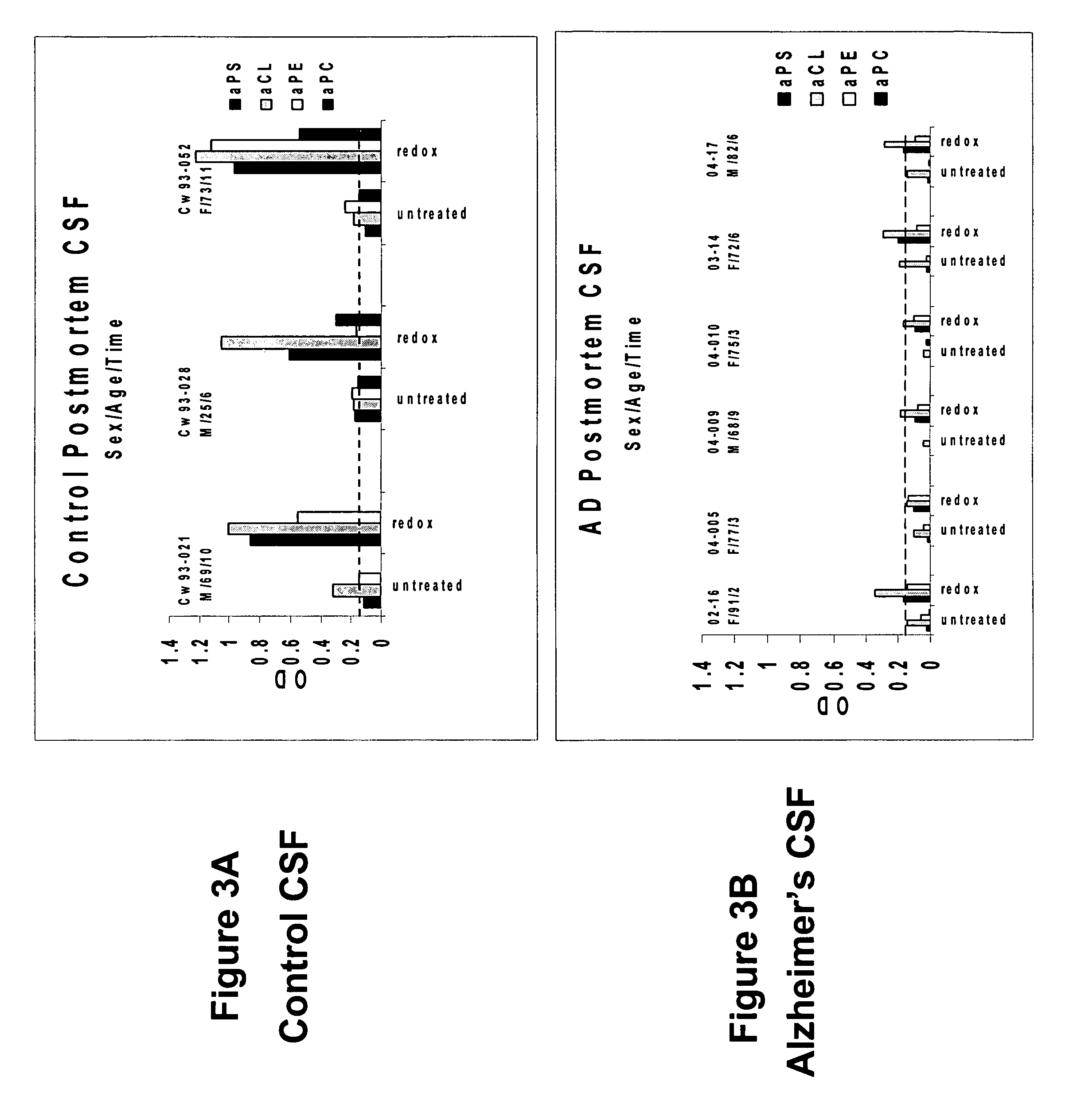
Method of detecting or diagnosing of a neurodegenerative disease or condition
A neurodegenerative disease or condition is diagnosed in a subject by obtaining a sample of cerebral spinal fluid from the subject and assaying the sample by an assay method that detects the presence of at least one antiphospholipid autoantibody in the sample, wherein an elevated level of at least one antiphospholipid autoantibody in the sample of cerebral spinal fluid correlates with a neurodegenerative disease or condition in the subject. The neurodegenerative disease or condition may also be diagnosed by assaying a sample of cerebral spinal fluid to detect nitrosylated antibodies, wherein an elevated level of nitrosylated antibodies correlates with a neurodegenerative disease or condition in said subject. A neurodegenerative disease or condition is also detected or diagnosed by assaying a first sample of cerebral spinal fluid from the subject to determine a level of at least one autoantibody having a selected specificity, treating a second sample of cerebral spinal fluid with an oxidizing agent and assaying the oxidized second sample to determine a level the at least autoantibody having the selected specificity, and comparing the level of the at least one autoantibody in the first sample with the level of the at least one autoantibody in the oxidized second sample, wherein a lack of increase in the level of the at least one autoantibody in the oxidized second sample as compared to the level of the at least one autoantibody in the first sample correlates with a neurodegenerative disease or condition in said subject.
Owner:REDOX REACTIVE REAGENTS LLC
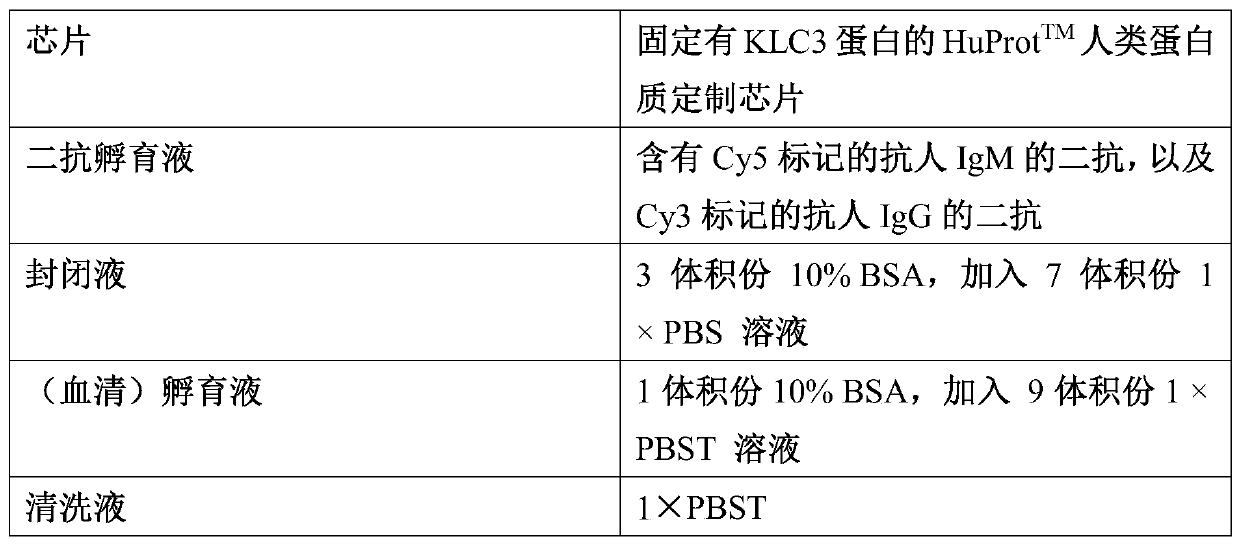
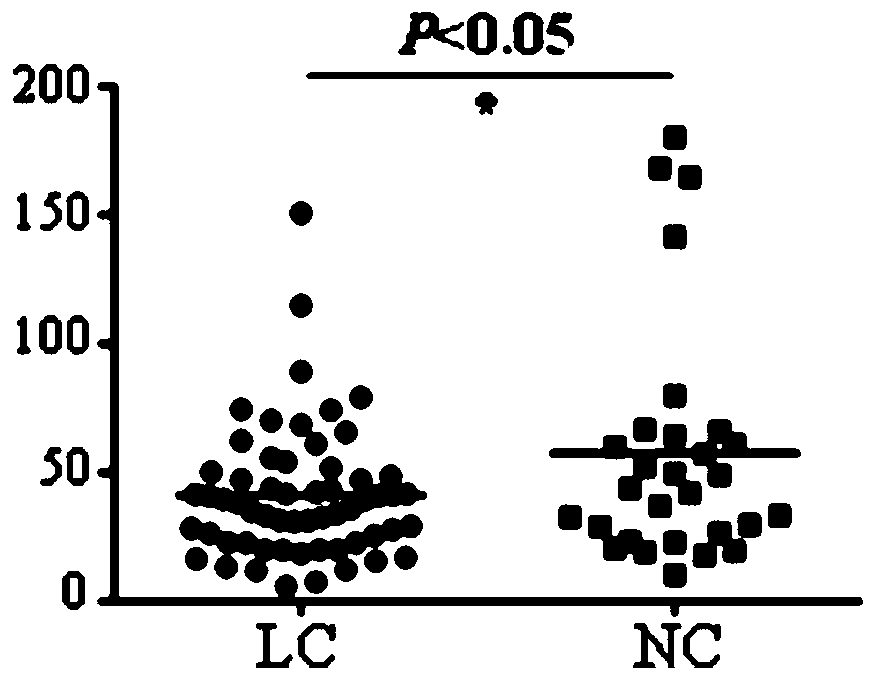
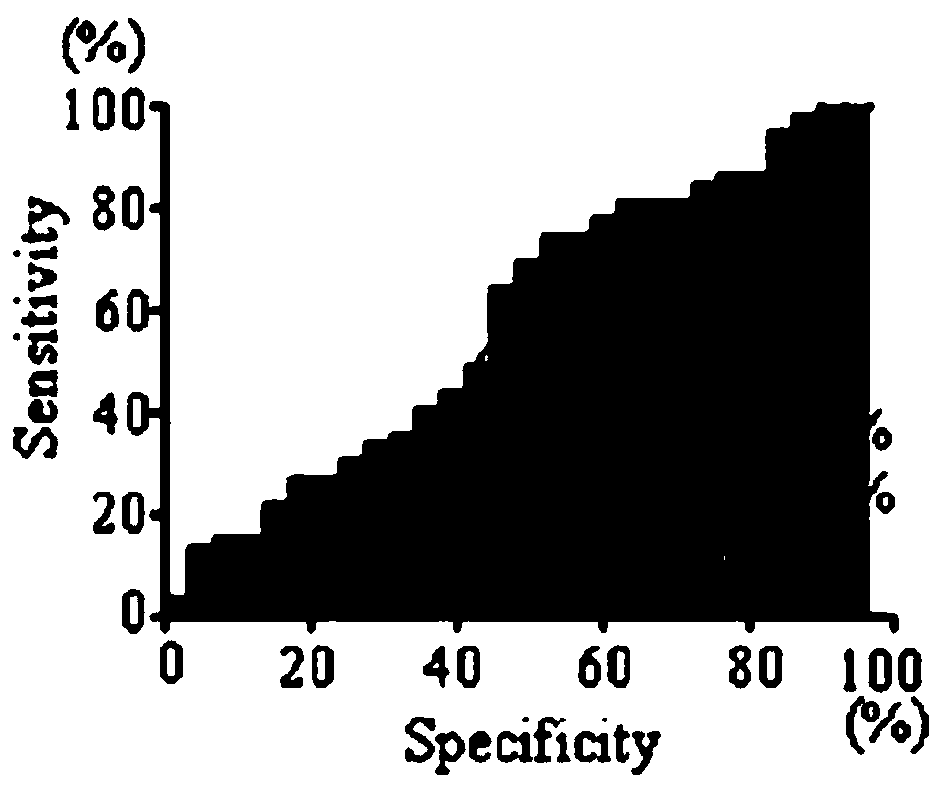
Application of KLC3 autoantibody detection reagent to preparation of lung cancer screening kit
The invention relates to the field of in-vitro diagnosis reagents, in particular to application of a KLC3 autoantibody detection reagent to preparation of a lung cancer screening kit. According to theapplication, it is discovered for the first time that the autoantibody level of KLC3 protein in serum of a lung cancer patient is remarkably higher than that of a healthy patient. Through the application, the reagent for detecting a KLC3 protein autoantibody is used for preparing the lung cancer screening kit, and effective screening of a lung cancer can be realized.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +1
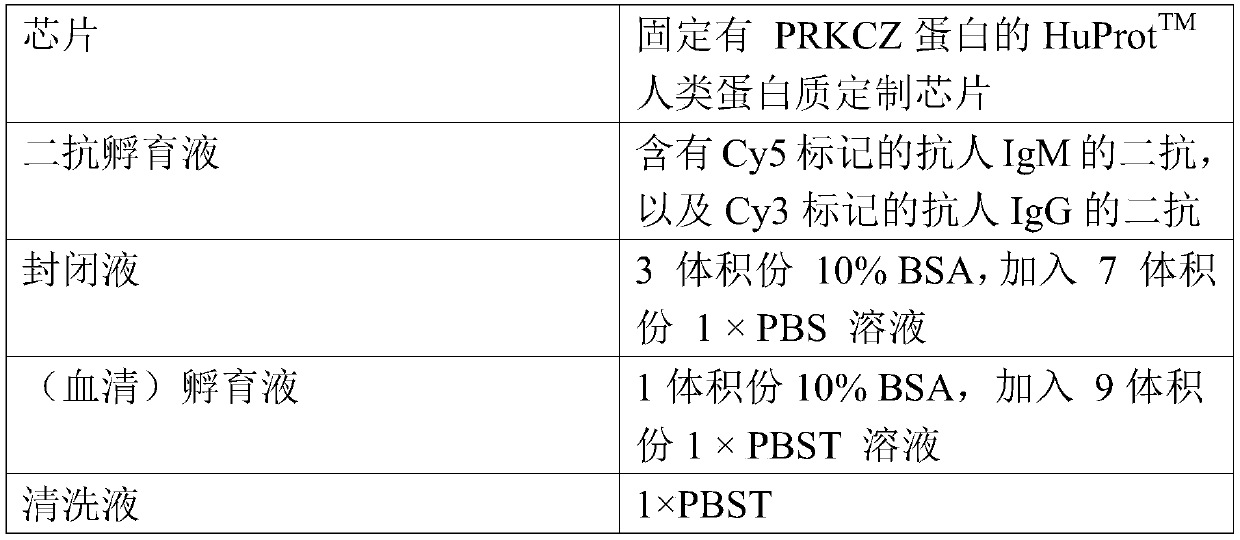
Application of PRKCZ autoantibody detection reagent in preparation of lung cancer screening kit
The invention relates to the field of in vitro diagnostic reagents, in particular to an application of a PRKCZ autoantibody detection reagent in preparation of a lung cancer screening kit. The invention finds for the first time that the autoantibody level of PRKCZ protein in serum of a lung cancer patient is significantly higher than that of a healthy patient. The reagent for detecting a PRKCZ protein autoantibody is used for preparing the lung cancer screening kit and effective screening of lung cancer can be realized.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
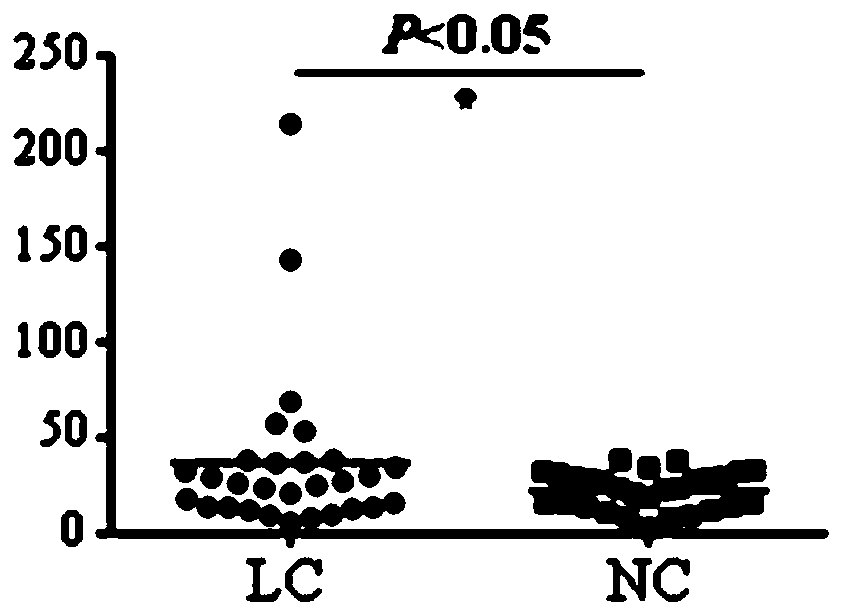
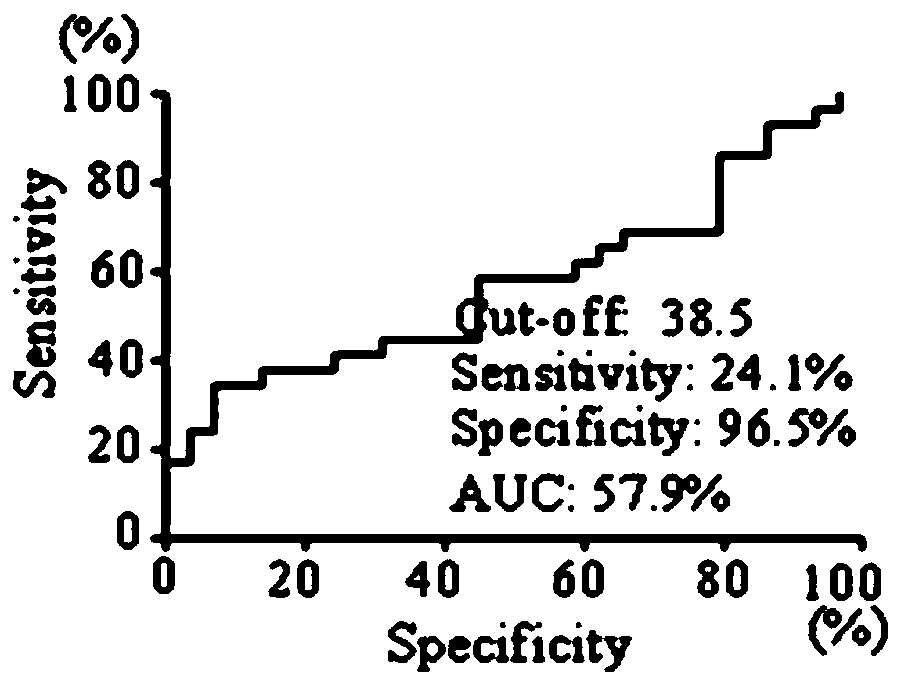
Application of EHD2 autoantibody detection reagent to preparation of lung cancer screening kit
The invention relates to the field of in-vitro diagnostic reagents and particularly relates to an application of an EHD2 autoantibody detection reagent to preparation of a lung cancer screening kit. According to the application in the invention, it is found for the first time that the autoantibody level of the EHD2 protein in the serum of a lung cancer patient is obviously higher than that of a healthy patient. According to the application in the invention, the reagent for detecting the autoantibody of the EHD2 protein is applied to preparation of the lung cancer screening kit, so that effective screening of the lung cancer can be realized.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +1
Measurement of PKA for cancer detection
ActiveUS8455200B2Low levelEasy to measurePeptide/protein ingredientsMicrobiological testing/measurementAnalyteMedicine
Owner:TRAXXSSON
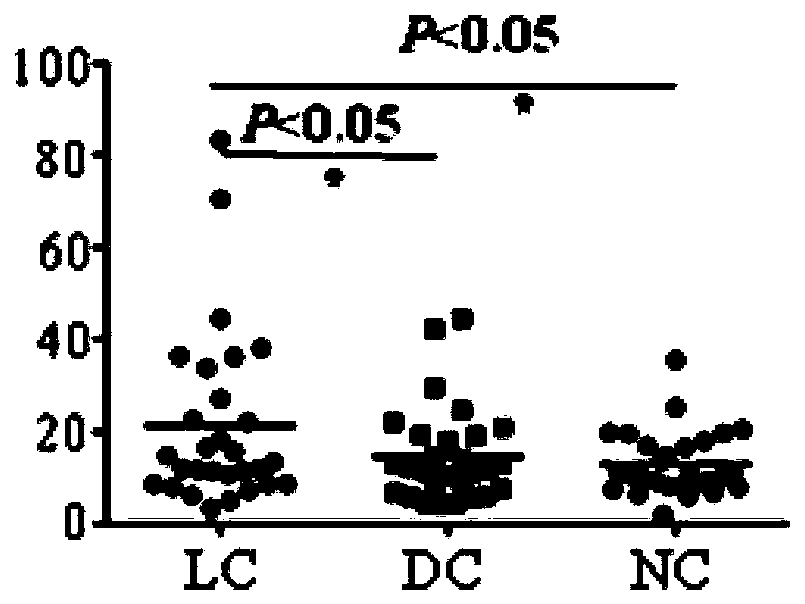
Biomarker panel for TA (Takayasu arteritis) detection and application of biomarker panel
The invention discloses a biomarker panel for TA (Takayasu arteritis) detection and an application of the biomarker panel to preparation of reagents for TA diagnosis. The biomarker panel totally contains 22 biomarkers, wherein a first group contains 13 target antigens which are used for differential diagnosis of TA and non-TA by detecting autoantibody level of SPATA7, IL17RB, ZFAND4, PRH2, CLPTM1,PGPEP1, DIXDC1, NDP, SLC27A2, PLA2G16, MAPK1IP1L, HSF2 and NOL3 in serum of a patient, accuracy is 83.6%, and sensitivity and specificity are 50.9% and 94.4% respectively; a second group contains 13target antigens which are used for distinguishing active phase from stationary phase of TA by detecting antibody level of QDPR, PKD1L2, ZFAND4, DIXDC1, NDP, PLA2G16, CIDEA, TRIM36, EIF3H, USF2, AGGF1,SPANXN2 and MCM3 in serum of a patient with TA, accuracy is 96.2%, and sensitivity and specificity are 96.19% and 96.4% respectively.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI +1
Antigen polypeptide of tumor inhibition factor p16, and application thereof
ActiveCN107163131AIncrease specific binding rateLow costDisease diagnosisBiological testingMultiple Tumor Suppressor-1Autoantibody production
The invention discloses an antigen polypeptide of a tumor inhibition factor p16, and an application thereof. The polypeptide active fragment of the antigen polypeptide of the tumor inhibition factor p16 is at least one of an amino acid sequence (1) represented by SEQ ID NO: 1 and an amino acid sequence (2) obtained through deleting, inserting or replacing the amino acid sequence (1) represented by SEQ ID NO: 1 and having the same functions. The antigen polypeptide can be applied to the preparation of a kit for detecting the p16 autoantibody level in a blood sample. The antigen polypeptide has a specific antigenic determinant, so the specific binding rate of the antigen polypeptide and the autoantibody of the tumor inhibition factor p16 contained in a sample to be tested is improved, and the inspection sensitivity and the detection efficiency are increased, thereby the risk of early tumors can be accurately predicated, and reliable data is provided for the study of new tumor drugs.
Owner:SHENZHEN HUAZHONG BIOLOGICAL PHARMA MACHINERY
Application of SDPR autoantibody detection reagent in preparing lung cancer screening kit
PendingCN110632311AEfficient screeningReduce harmBiological material analysisBiological testingSerum igeAutoantibody production
The present invention relates to the field of in vitro diagnostic reagents, and in particular to the application of an SDPR autoantibody detection reagent in preparing a lung cancer screening kit. Inthe present invention, that the autoantibody level of the SDPR protein in the serum of lung cancer patients is significantly higher than that of healthy patients is discovered for the first time. According to the technical scheme of the present invention, the SDPR protein autoantibody detection reagent is used to prepare a lung cancer screening kit, and effective lung cancer screening can be realized.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
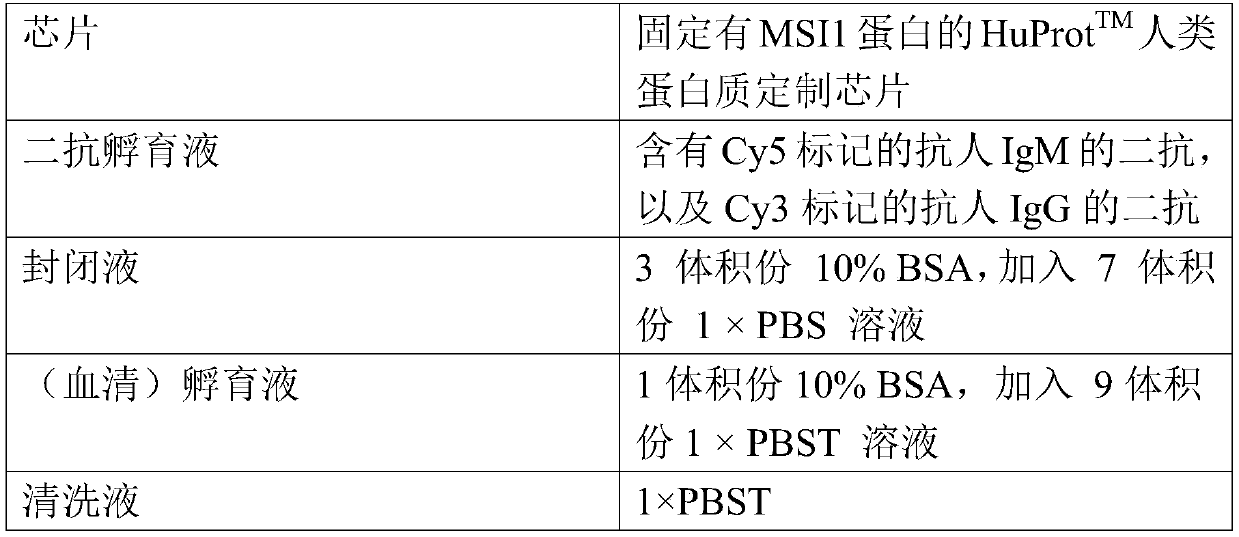
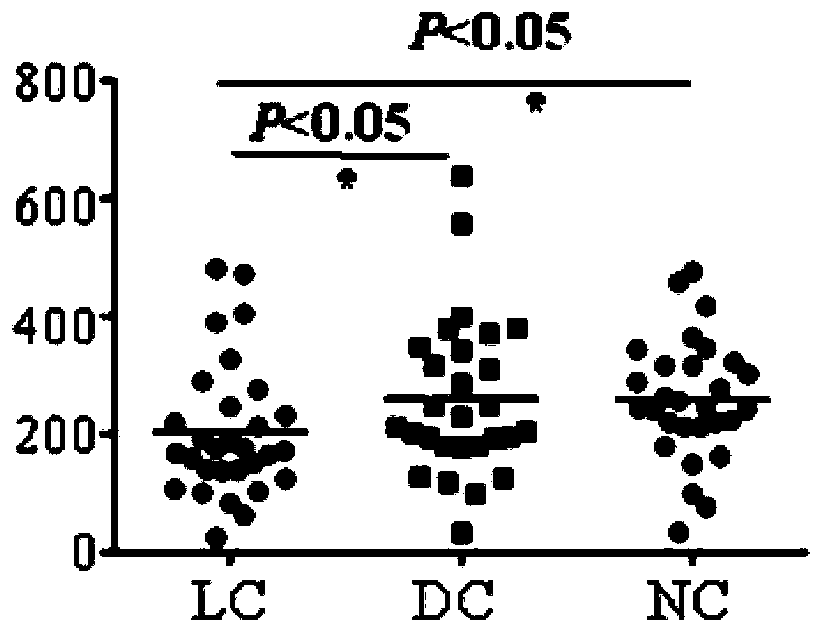
Use of detection reagent of MSI1 autoantibody in preparation of lung cancer screening kit
PendingCN110632315AEfficient screeningReduce harmBiological material analysisBiological testingSerum igeAutoantibody production
The invention relates to the field of in vitro diagnostic reagents, and in particular to use of a detection reagent of a MSI1 autoantibody in preparation of a lung cancer screening kit. The inventionfinds that the level of autoantibody of MSI1 protein in the serum of lung cancer patients is significantly lower than that in healthy patients for the first time, and can realize effective screening of lung cancer by using the reagent of detecting the autoantibody of MSI1 protein for the preparation of a lung cancer screening kit.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Application of EEF2K (Eukaryotic Elongation Factor 2 Kinase) autoantibody detection reagent to preparation of lung cancer detection kit
ActiveCN110596390AEfficient screeningReduce harmMaterial analysisSerum igeEukaryotic Elongation Factor-2 Kinase
The invention relates to the field of in-vitro diagnosis reagents, in particular to application of an EEF2K (Eukaryotic Elongation Factor 2 Kinase) autoantibody detection reagent to the preparation ofa lung cancer screening kit. The invention first discovers that the autoantibody level of EEF2K proteins in the serum of a lung cancer patient is remarkably higher than that of a healthy patient. Thereagent for detecting the EEF2K protein autoantibody is used for preparing the lung cancer screening kit, so that the effective screening of the lung cancer can be realized.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
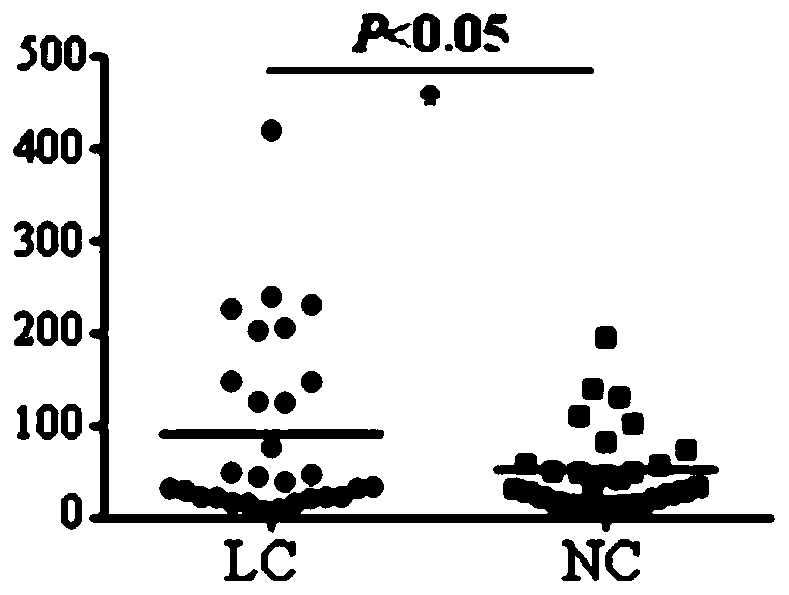
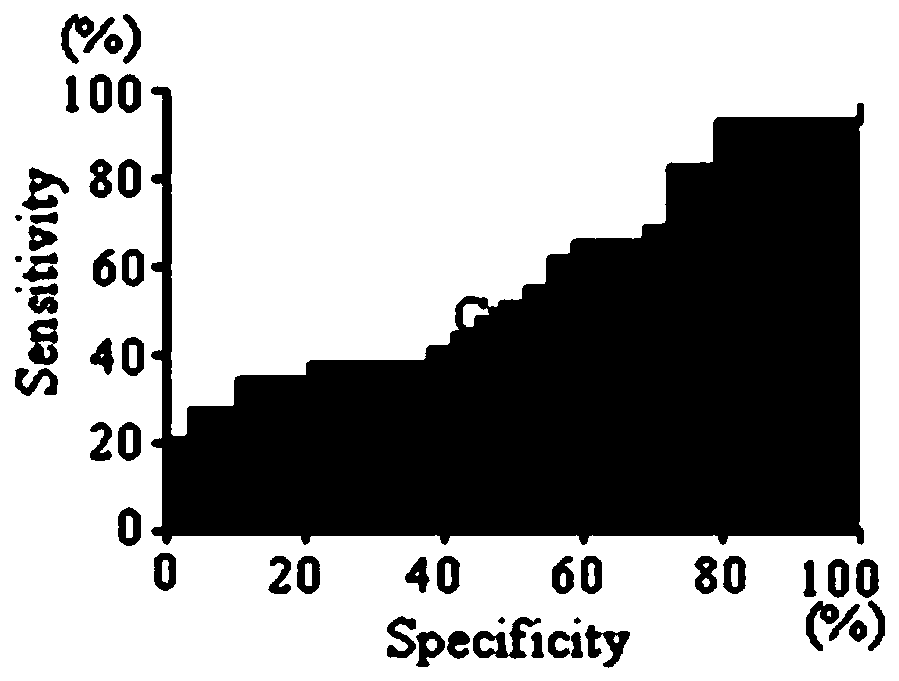
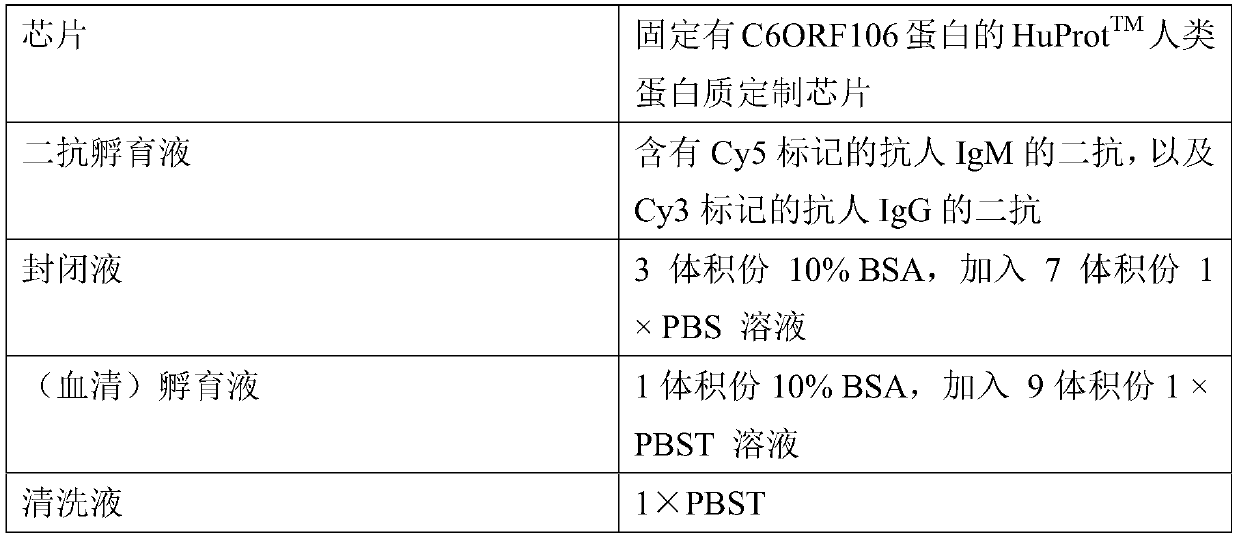
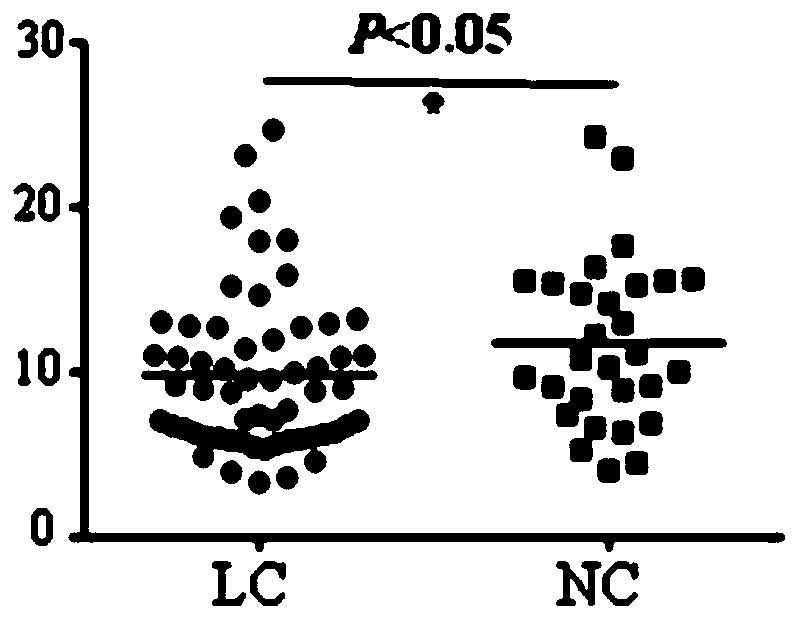
Application of C6ORF106 antibody detection reagent in fabrication of lung cancer screening kit
The invention relates to the field of in-vitro diagnostic reagent, in particular to application of a C6ORF106 antibody detection reagent in fabrication of a lung cancer screening kit. The antibody level of C6ORF106 protein in serum of a lung cancer patient is initially found out to be remarkably higher than that of a health person. The reagent for detecting the C6ORF106 protein antibody is used for fabricating the lung cancer screening kit, and effective screening of lung cancer can be achieved.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +1
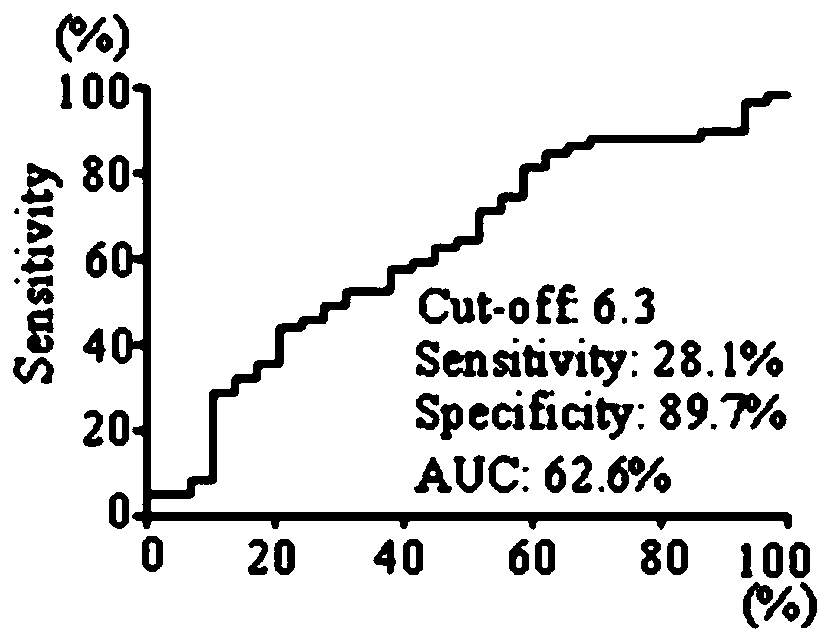
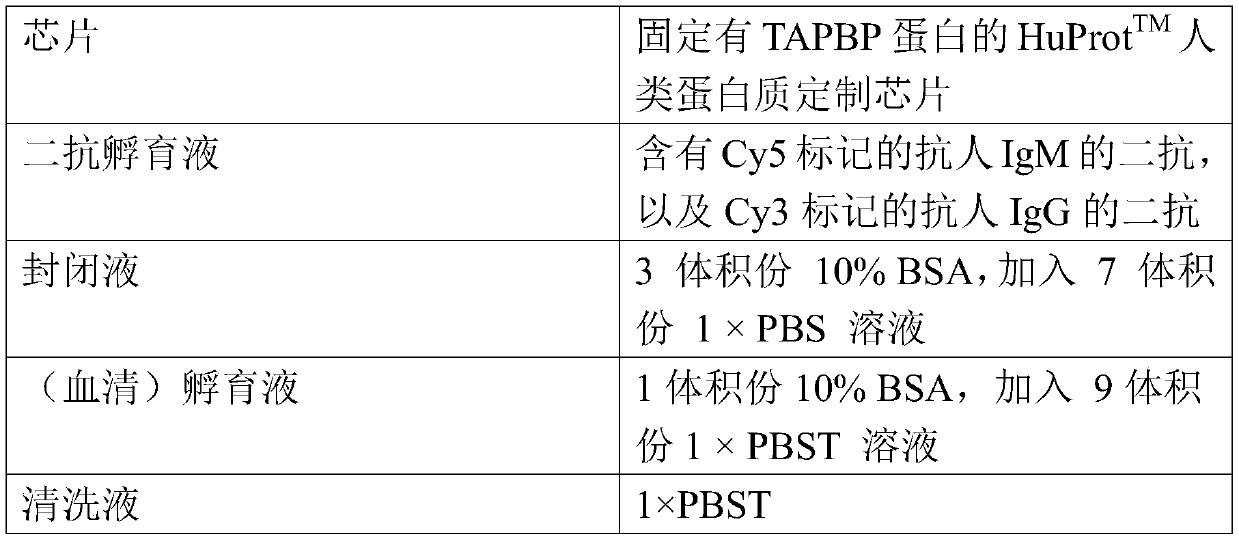
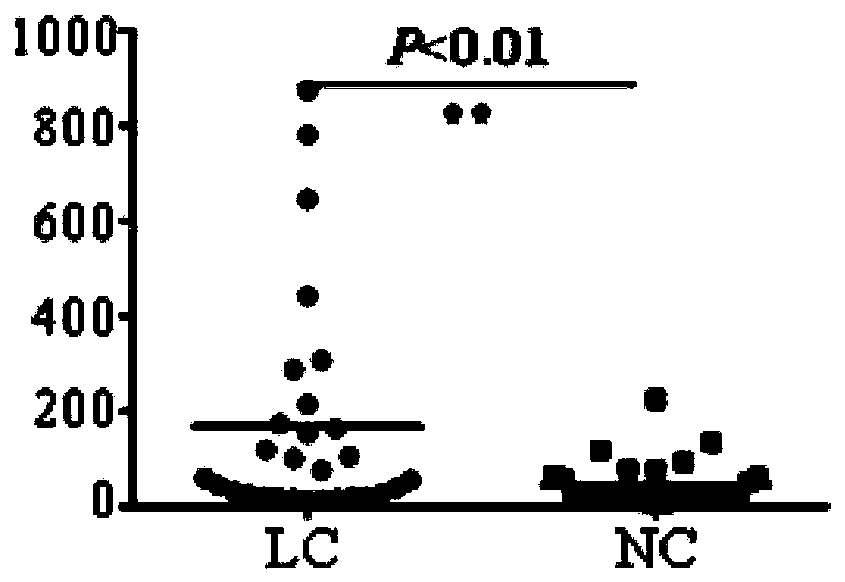
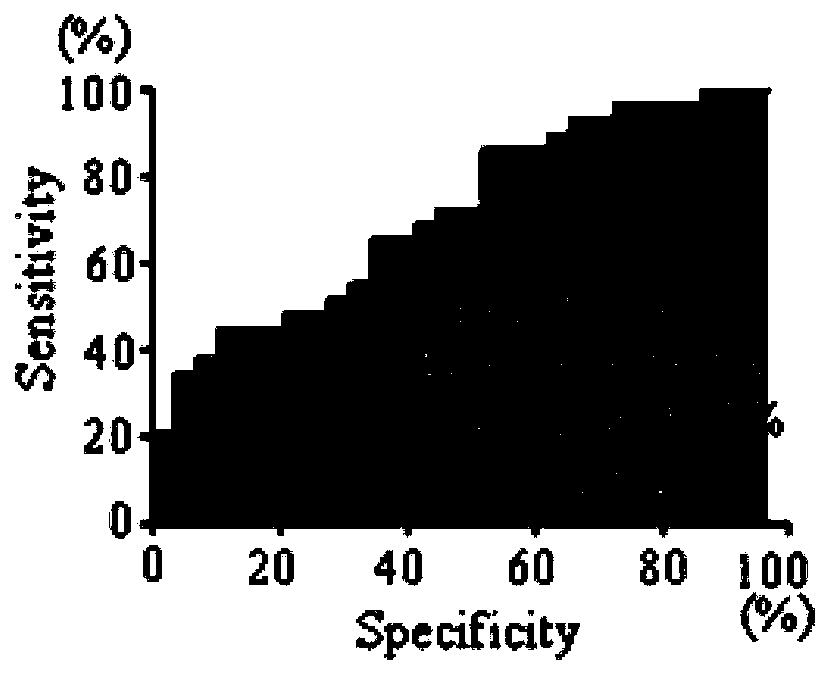
Application of TAPBP autoantibody detection reagent in preparation of lung cancer screening kit
The invention relates to the field of in vitro diagnostic reagents, in particular to an application of a TAPBP autoantibody detection reagent in preparation of a lung cancer screening kit. The invention finds for the first time that the autoantibody level of TAPBP protein in serum of a lung cancer patient is significantly higher than that of a healthy patient. The reagent for detecting a TAPBP protein autoantibody is used for preparing the lung cancer screening kit and effective screening of lung cancer can be realized.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Use of DBNL autoantibody detection reagent in preparation of lung cancer screening kit
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +1
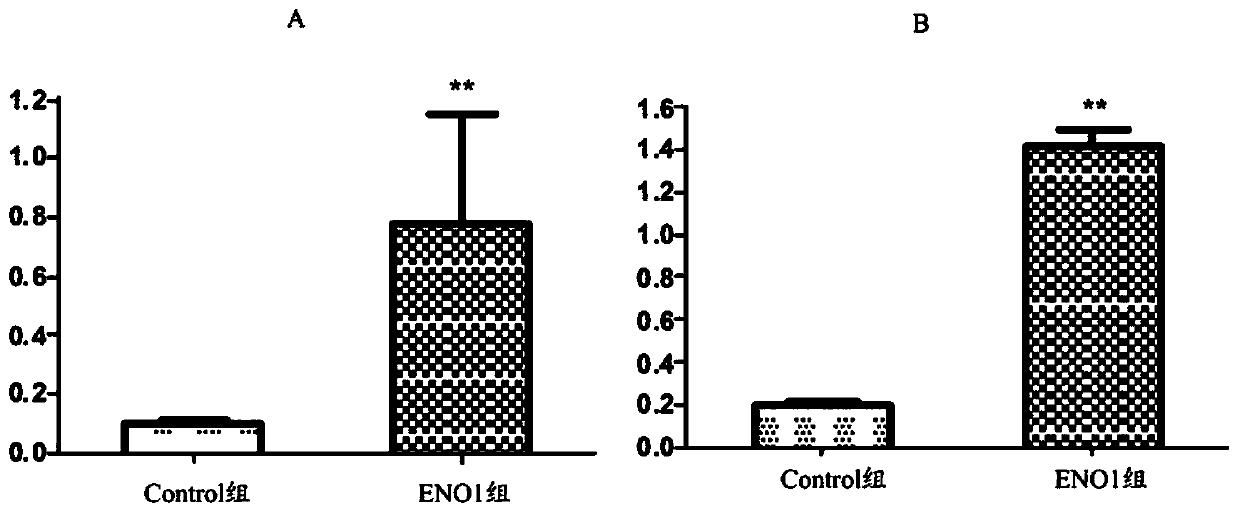
Application of anti-eno1 autoantibodies in screening pregnant women with AIT and predicting the risk of miscarriage
ActiveCN107991493BEffectively predict the risk of miscarriageReduce miscarriageDisease diagnosisBiological testingAutoantibodyPhysiology
The invention belongs to the technical field of biomedicine, and concretely discloses application of predicting abortion risk of a pregnant woman suffering from AIT (autoimmune thyroiditis) through detecting the ENO1 (alpha-enolase) specific autoantibody (ENO1Ab) level in serum. The invention discovers that the existence of the ENO1Ab is closely related to the occurrence of abortion and the attackof placenta dysfunction, so that novel clinical application for timely screening out the AIT pregnant woman with high abortion risk is provided.
Owner:THE FIRST HOSPITAL OF CHINA MEDICIAL UNIV
Use of CCDC130 autoantibody detection reagent in preparation of lung cancer screening kit
The invention relates to the field of in vitro diagnostic reagents, and in particular relates to use of a CCDC130 autoantibody detection reagent in preparation of a lung cancer screening kit. The factthat the autoantibody level of CCDC130 protein in the serum of a patient with lung cancer is obviously higher than that of a health patient is found in the invention for the first time. The reagent for detecting a CCDC130 protein autoantibody is used for preparing the lung cancer screening kit in the invention; and thus, effective lung cancer screening can be realized.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +1
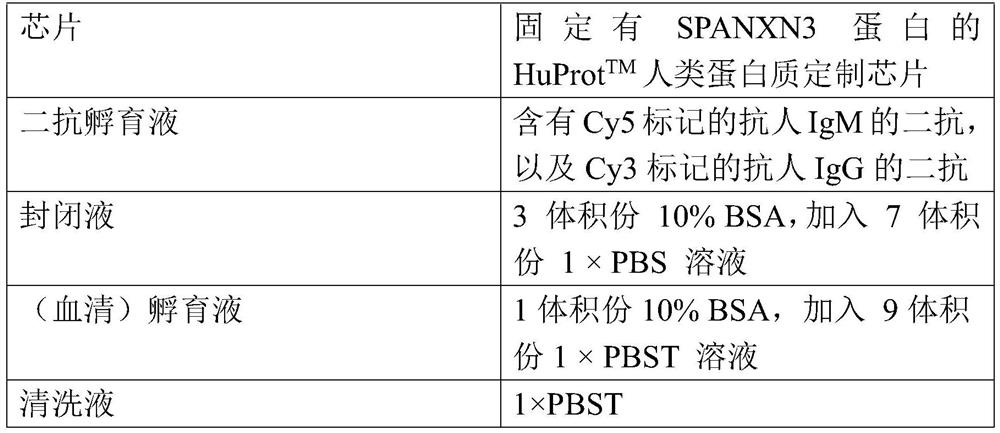
Use of the spanxn3 autoantibody detection reagent in the preparation of a lung cancer screening kit
The invention relates to the field of in vitro diagnostic reagents, in particular to the use of SPANXN3 autoantibody detection reagents in the preparation of lung cancer screening kits. The present invention finds for the first time that the autoantibody level of SPANXN3 protein in serum of patients with lung cancer is significantly lower than that of healthy patients. The invention uses the reagent for detecting SPANXN3 protein autoantibody to prepare a lung cancer screening kit, which can realize effective screening of lung cancer.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com