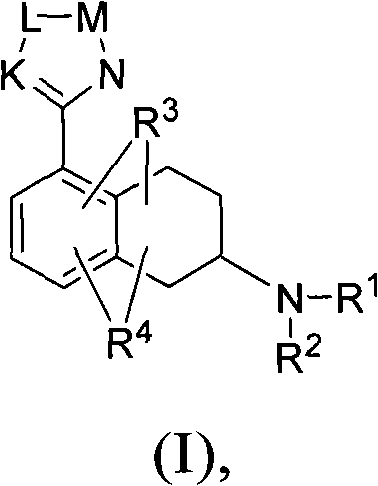
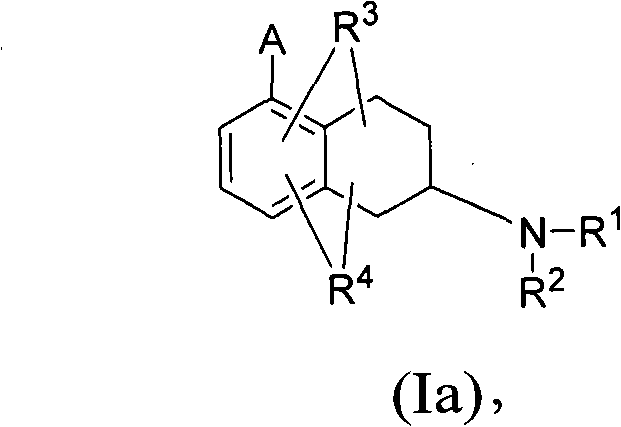
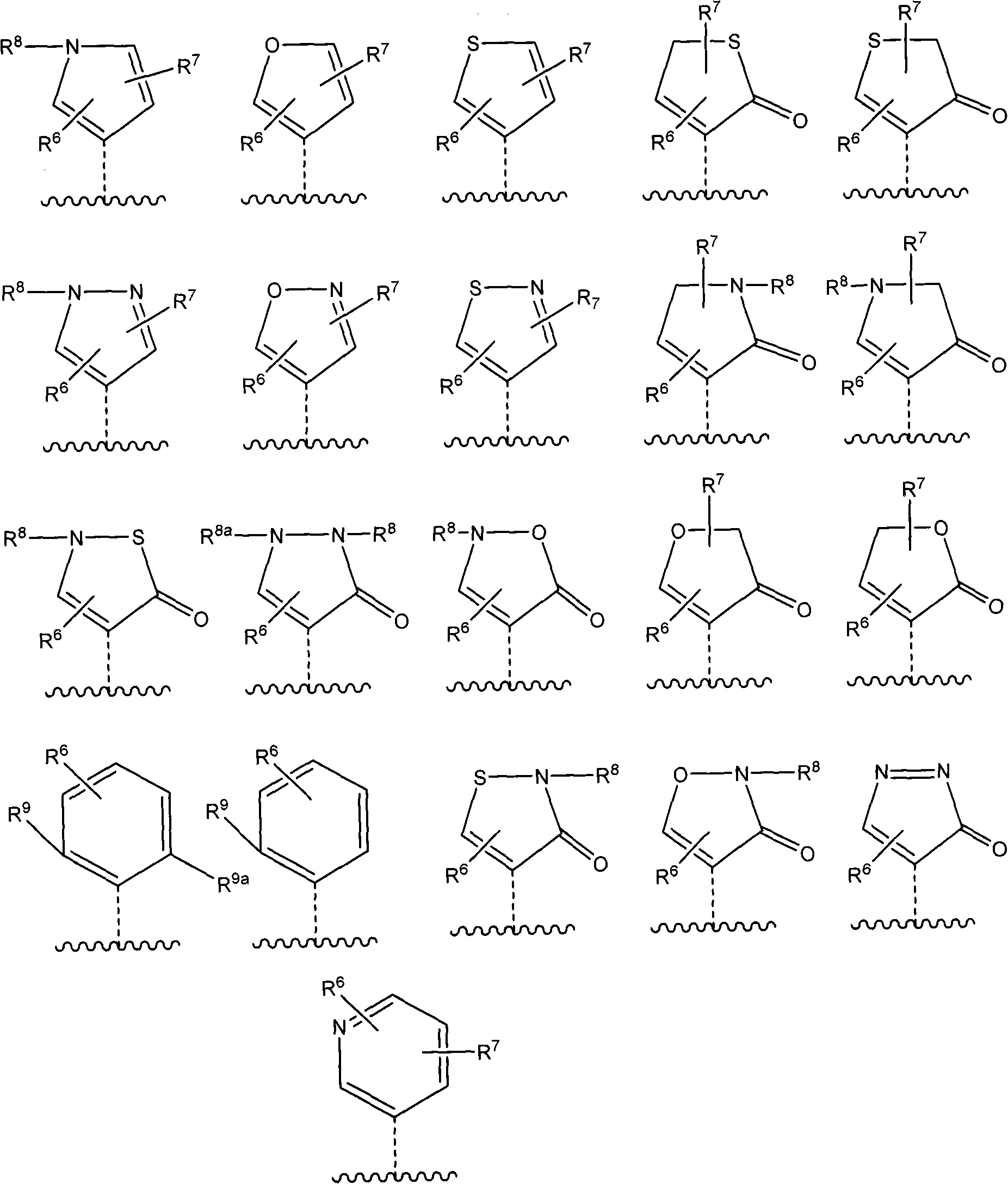
Heterocyclyl-substituted-tetrahydro-naphthalen-amine derivatives, their preparation and use as medicaments
A technology of amine derivatives and heterocyclic groups, applied in the field of tetralin amine compounds, can solve problems such as low sequence homology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
[0476] N-(5-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)-N,N-dimethylamine
[0477]
[0478] To dissolve in CH 2 Cl 2 To a solution of 5-methoxy-2-tetralone (10.33 g, 58.62 mmol) in (400 mL) was added dimethylamine (5.6 M in EtOH, 14 mL, 76.206 mmol) and AcOH (0.46 mL, 5.862 mmol) ), and the mixture was stirred at room temperature for 4 hours. It was then cooled to 0 °C and NaB(OAc) was added within 20 min 3 H (0.45eq (equiv), 5.59g, 26.379mmol). After stirring at 0°C for 1 hour, NaB(OAc) was added within 30 min 3 H (1.0 eq, 12.42 g, 58.62 mmol). The reaction mixture was warmed to room temperature and stirred for 16h. The mixture was cooled to 0 °C again, and H was added slowly 2 O (250 mL). By adding NaHCO 3 Saturated aqueous solution, adjust the pH of the solution to 8.0, and stir the mixture at 0 °C for 15 min. layered, with CH 2 Cl 2 (4 x 100 mL) to extract the aqueous phase. Combine all organic phases and wash with anhydrous Na 2 SO 4 Dry and concentrate i...
Embodiment B
[0481] 6-(Dimethylamino)-5,6,7,8-tetralin-1-ol
[0482]
[0483] N-(5-Methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)-N,N-dimethylamine (8.86 g, 43.156 mmol) was dissolved in CH 2 Cl 2 (200mL), cooled to 0°C, and added BBr within 20 minutes 3 (CH 2 Cl2 1.0M, 51.8mL, 51.788mmol). The reaction mixture was allowed to reach room temperature by stirring overnight (ca. 14h). The mixture was cooled to 0 °C again and NH was added slowly 3 aqueous solution (25%, 50 mL), and the mixture was stirred at 0° C. for 15 minutes. The salt was filtered off, the layers were separated, and the 2 Cl 2 (4 x 40 mL) to extract the aqueous phase. Combine all organic phases, wash with anhydrous Na 2 SO 4 Dry and concentrate in vacuo. The residue (6.99g) was dissolved in silica gel (30:70:2-100:0:2AcOEt / hexane / Et 3 N and 30:70:2 AcOEt / Hexane / Et 3 N-90:10:2AcOEt / MeOH / Et 3 Purification by flash chromatography on N) afforded 2.80 g of the title compound (Rf=0.3 (AcOEt / hexane / Et 3 N 10:10:2), m...
Embodiment C
[0486] N-Benzyl-N-(5-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)amine
[0487]
[0488] To dissolve in CH 2 Cl 2 Benzylamine (23 mL, 212.80 mmol) and AcOH (0.97 mL, 17.02 mmol) were added to a solution of 5-methoxy-2-tetralone (30 g, 170.24 mmol) in (250 mL), and the mixture was stirred at room temperature for 4 h. Then cool to 0°C and add NaB(OAc) within 20min 3 H (0.38eq, 13.71g, 64.69mmol). After stirring at 0°C for 1 hour, NaB(OAc) was added within 30 min 3 H (1.07eq, 38.61g, 182.16mmol). Join CH 2 Cl 2 (100 mL), the reaction mixture was warmed to room temperature and stirred for 15 h. The mixture was cooled to 0 °C again, and H was added slowly 2 O (200 mL). By adding NaHCO 3 Saturated aqueous solution (300 mL), the pH of the solution was adjusted to 8.0, and the mixture was stirred at 0° C. for 15 min. layered, with CH 2 Cl 2 (2 x 150 mL) to extract the aqueous phase. Combine all organic phases, wash with anhydrous Na 2 SO 4 Dry and concentrate in vacuo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com