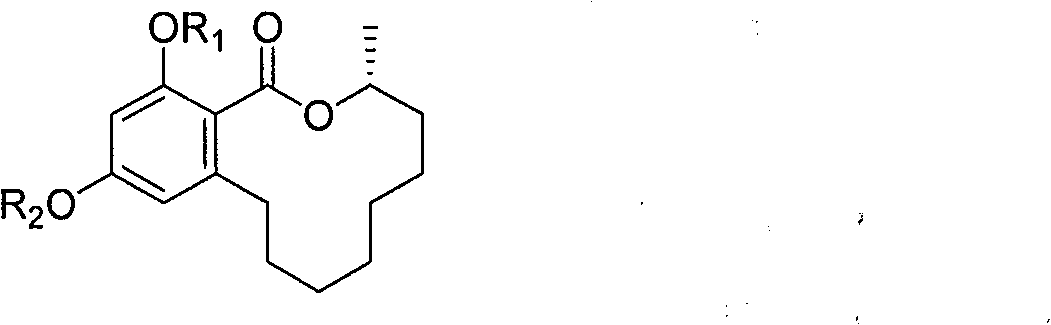
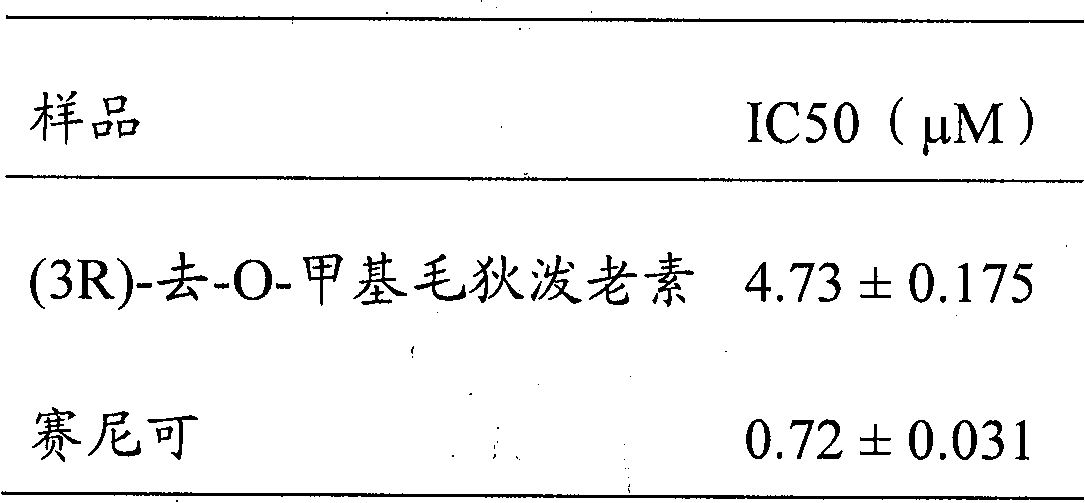
Benzo macrolide compound (3R)- des-O-methyllasiodiplodin, its derivatives and preparation method and use
A technology of methylmadiprene and macrolides, which is applied in the field of benzomacrolides and can solve problems such as no biological activity research reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of benzomacrolide compound (3R)-des-O-methylmadiprelline
[0025] (1) Extraction: The dry weight of the mangrove plant Mango chinensis is 3.1kg, and it is extracted 4 times by percolation with 10L of methanol. The extracts are combined and then concentrated under reduced pressure. The obtained extract is suspended in 1000ml of 1N NaCl solution, and extracted repeatedly with 1000ml of petroleum ether. The suspension was collected three times, and the resulting extracts were combined and concentrated under reduced pressure to obtain 11 g of petroleum ether extract.
[0026] (2) Separation: 11g of petroleum ether extract was subjected to 100-200 mesh silica gel column chromatography, and petroleum ether / ethyl acetate 100:0→95:5→90:10→70:30→50:50→30:70 → 0:100 gradient elution, the amount of each gradient is 1000ml; among them, 1.2g of the eluted part with a volume ratio of petroleum ether / ethyl acetate of 60:40 is washed with a gradient of petroleum ether / ace...
Embodiment 2
[0029] (3R)-Methylate (R 1 and R 2 are all methyl) prepare
[0030] Weigh 5.0 mg of the (3R)-de-O-methylmaudeprene sample prepared in Example 1 in a 25 mL round bottom flask, add 2 mL of NaOH solution dissolved with 2.5 times the equivalent of methyl iodide, and add an appropriate amount of phase Transfer catalyst tetrabutylammonium bromide, heat and reflux in 10mL water / dichloromethane two-phase system for 4 hours to obtain the methylate (R 1 =R 2 =CH 3 ).
[0031] (3R)-De-O-methylmadipredine methylated spectral data are as follows: C 18 h 28 o 4 ; 1 HNMR (300MHz, CDCl 3) 6.24(d, J=2.6Hz, H-5′), 6.21(d, J=2.6Hz, H-3′), 5.18(m, H-3), 3.65, 3.61 (both s, 2×OCH 3 ), 3.26, 2.51 (m, H-10), 1.40~2.49 (H-4~9), 1.37 (d, J=7.0Hz, 3-Me).
Embodiment 3
[0033] (3R)-Acetylate (R 1 and R 2 are all acetyl) preparation
[0034] Weigh 5.0 mg of the (3R)-des-O-methylmaudeprene sample prepared in Example 1 and dissolve it in 4 mL of pyridine solution, add 2 mL of acetic anhydride solution, and react overnight at room temperature to obtain (3R)-des- The acetylated compound of O-methylmadipredrine (R 1 = R 2 =COCH 3 ).
[0035] (3R)-De-O-methylmadipredine acetylate spectral data are as follows: C 20 h 28 o 6 ; 1 HNMR (300MHz, CDCl 3 ) 6.24(d, J=2.6Hz, H-5'), 6.21(d, J=2.6Hz, H-3'), 5.18(m, H-3), 3.26, 2.51(m, H-10), 2.28, 2.26 (both s, 2×COCH 3 ) 1.40~2.49 (H-4~9), 1.37 (d, J=7.0Hz, 3-Me).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com