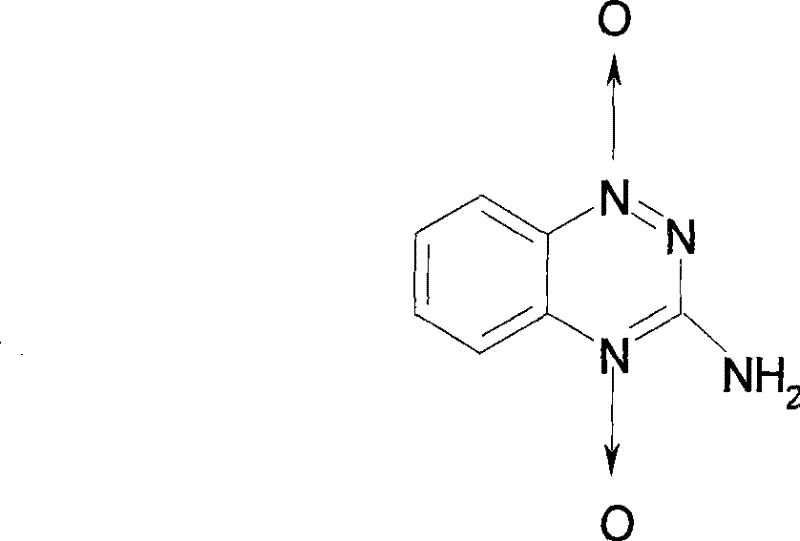
Tirapazamine parenteral hydrous preparation and preparation method thereof
A technology for tirapazamine and intestinal tract, which is applied in the field of tirapazamine parenteral administration aqueous preparations and its preparation, and can solve the problems such as easy precipitation of tirapazamine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
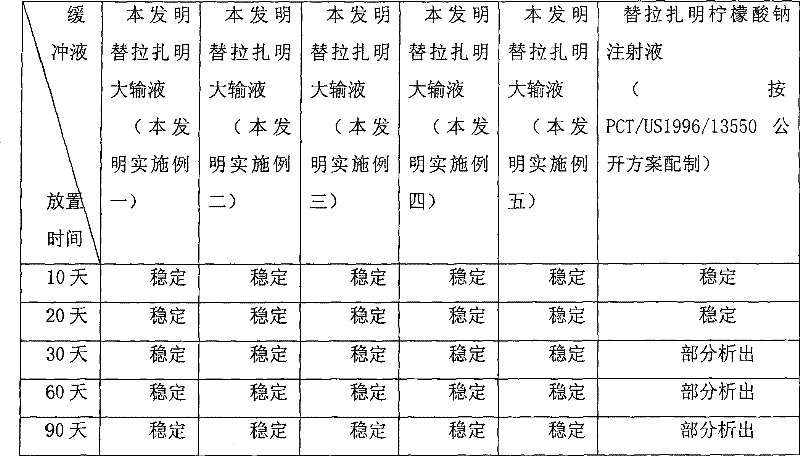
Image
Examples
preparation example 1
[0036] a: tirapazamine 0.01 g
[0037] b: citric acid 0.0042 g
[0038] c: 0.001 g of sodium hydroxide
[0039] Take 300 ml of water to dissolve b and c. After complete dissolution, raise the temperature appropriately to keep the solution temperature at about 60°C, slowly add a, stir to dissolve it completely, cool to room temperature, add sodium chloride and other osmotic pressure regulators, add appropriate amount of water to 1000 ml, and the pH is about is 6.5.
[0040] Preparation administration method: intravenous infusion.
preparation example 2
[0042] a: Tirapazamine 0.16g
[0043] b: citric acid 0.0208g
[0044] c: 0.00594 grams of sodium hydroxide
[0045] Take 300 ml of water to dissolve b and c. After complete dissolution, raise the temperature appropriately to keep the solution temperature at about 50°C, slowly add a, stir to dissolve it completely, after cooling to room temperature, add sodium chloride and other osmotic pressure regulators, add appropriate amount of water to 1000 ml, and the pH is about is 3.0.
[0046] Preparation administration method: intravenous infusion.
preparation example 3
[0048] a: tirapazamine 0.01 g
[0049] b: citric acid 0.0168g
[0050] c: 0.004 grams of sodium hydroxide
[0051] Take 300 ml of water to dissolve b and c. After complete dissolution, heat up appropriately to keep the solution temperature at about 55°C, slowly add a, stir to dissolve completely, after cooling to room temperature, add glucose and other osmotic pressure regulators, add appropriate amount of water to 1000 ml, pH is about 5.3 .
[0052] Preparation administration method: intravenous infusion.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com