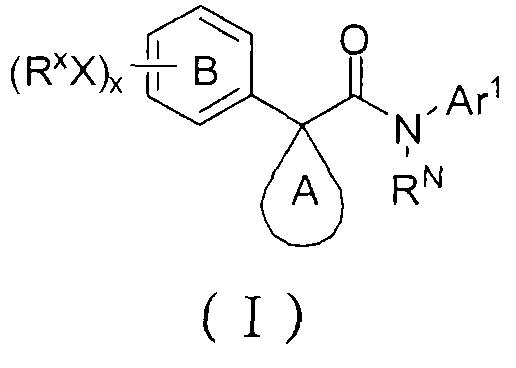
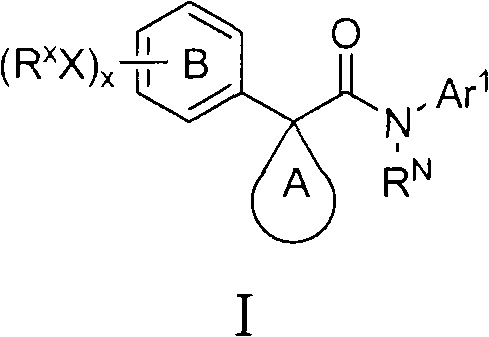
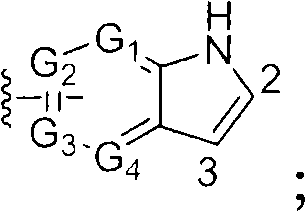
Azaindole derivatives as cftr modulators
A compound, heteroaryl technology, applied in the field of regulators, can solve problems such as increasing disease severity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0172] Table 2 below contains a listing of carboxylic acid synthesis materials that are commercially available or prepared by one of the methods described below.
[0173] compound
[0174]The mixture was stirred at 70°C overnight, then the reaction mixture was diluted with water (30 mL) and extracted with ethyl acetate. The combined organic layers were dried over sodium sulfate and evaporated to dryness to yield crude 1-(2,2-difluoro-benzo[1,3]dioxol-5-yl)-cyclopropanenitrile, which was used directly in subsequent steps. Step f: 1-(2,2-difluoro-benzo[1,3]dioxol-5-yl)-cyclopropanecarboxylic acid [0007] converts 1-(2,2-difluoro- Benzo[1,3]dioxol-5-yl)-cyclopropanenitrile (crude product from previous step) was refluxed in 10% aqueous sodium hydroxide (50 mL) for 2.5 hours. The cooled reaction mixture was washed with diethyl ether (100 mL), and the aqueous phase was acidified to pH 2 with 2M hydrochloric acid. Filtration of the precipitated solid yielded 1-(2,2-difluo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com