Preparation method of N,N-diisopropylethylamine
A technology of diisopropylethylamine and diisopropylamine, applied in the N field, can solve the problems of high toxicity of diethyl sulfate, environmental pollution, etc., and achieve the advantages of reducing the burden of three wastes treatment, reducing costs, and reducing harm to the environment and human body. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
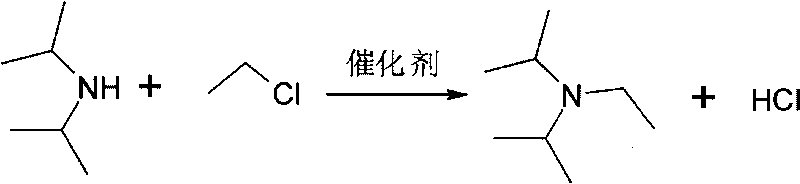
[0017] Embodiment 1, a kind of preparation method of N, N-diisopropylethylamine, take diisopropylamine and ethyl chloride as starting raw material, carry out the following steps successively:
[0018] Add 84 ml (0.6 mol) of diisopropylamine, 20.0 g (0.3 mol) of ethyl chloride, and 2 g of KI into an autoclave with a stirring temperature measuring device, and close the lid of the autoclave. use N 2 After leak detection and replacement several times, the temperature was raised to 130°C and the pressure was 0.8MPa. After maintaining the temperature for 10 h, the pressure of the autoclave no longer dropped, and the reaction was terminated.
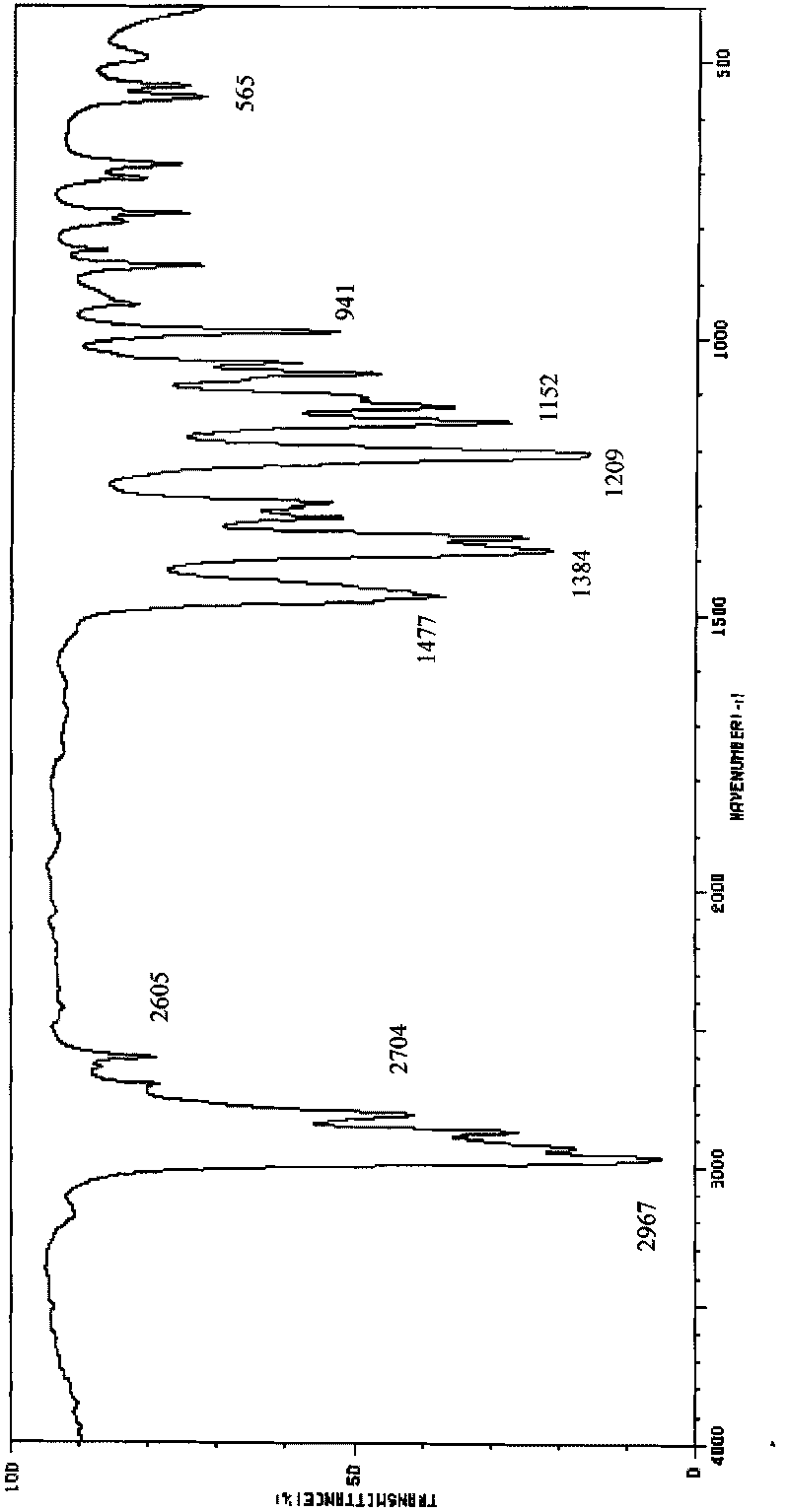
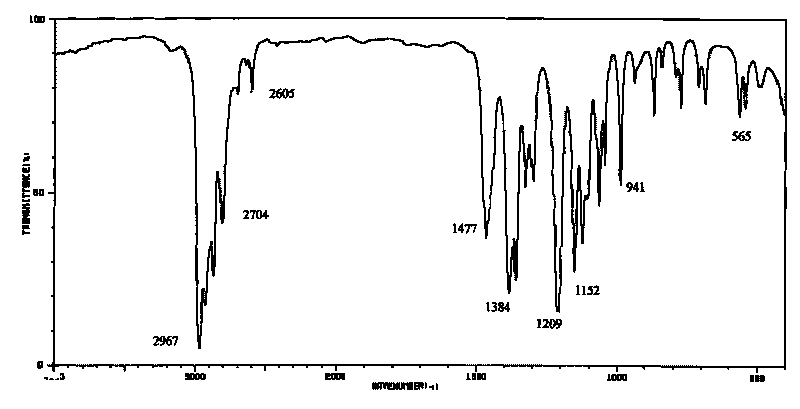
[0019] Add saturated NaOH solution to the obtained reaction solution until PH = 13, separate the organic phase (located on the upper layer) and carry out batch rectification at atmospheric pressure, collect fractions at 127.5-128.0°C, and obtain the product N, N-diisopropylethylamine 30.4 g, the yield is 78.5%, and the structure of the obtain...
Embodiment 2
[0020] Embodiment 2, a kind of preparation method of N, N-diisopropylethylamine, take diisopropylamine and ethyl chloride as starting raw material, carry out the following steps successively:
[0021] Add 84 ml (0.6 mol) of diisopropylamine, 20.0 g (0.3 mol) of ethyl chloride, and 2 g of NaI into a high-pressure reaction kettle with a stirring temperature measuring device, and close the lid of the kettle. use N 2 After leak detection and replacement several times, the temperature was raised to 150°C and the pressure was 1.0MPa. After maintaining the temperature for 8 hours, the pressure of the autoclave no longer dropped, and the reaction was terminated.
[0022] Add KOH solution to the resulting reaction solution until PH = 13, separate the organic phase and carry out batch rectification at atmospheric pressure, collect fractions at 127.5 to 128.0 ° C to obtain 31.3 g of the product N, N-diisopropylethylamine, and the yield is 80.6%, the structure of the obtained product wa...
Embodiment 3
[0023] Embodiment 3, a kind of preparation method of N,N-diisopropylethylamine, take diisopropylamine and ethyl chloride as starting raw material, carry out the following steps successively:
[0024] Add 127ml (0.9mol) of diisopropylamine, 20.0g (0.3mol) of ethyl chloride, CaI 2 1.5g, close the kettle lid. After leak detection and replacement with N2 several times, the temperature was raised to 150°C and the pressure was 1.2MPa. After maintaining the temperature for 8 hours, the pressure of the autoclave no longer dropped, and the reaction was terminated.
[0025] Add NaOH solution to the resulting reaction solution until PH = 13, separate the organic phase and carry out batch rectification at atmospheric pressure, collect fractions at 127.5 to 128.0°C to obtain 32.3 g of the product N,N-diisopropylethylamine, and the yield is 83.4%, the structure of the obtained product was confirmed by infrared spectroscopy.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com