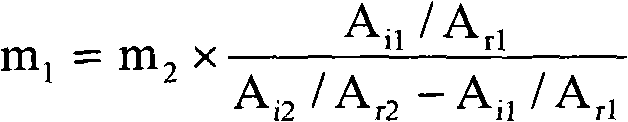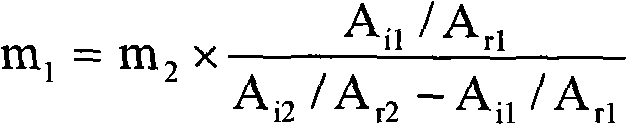Method for quantitatively detecting dermatan sulfate in heparin
A quantitative detection method, the technology of dermatan sulfate, applied in the field of biomedicine, can solve the problems of medication risk, repeated medication, inaccurate heparin measurement, etc., and achieve the effect of improving safety and controlling content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0022] The concrete operation of dermatan sulfate testing method of the present invention comprises the following steps:
[0023] About 0.4 g of the sample was weighed, diluted with water to a concentration of 10% (m / m), and 0.02 g of sodium nitrite was added. Use 6mol / L hydrochloric acid to adjust the pH of the solution to 1-1.5, and stir for 1.5-2 hours. Use 20% NaOH solution to adjust the pH of the solution to 6.5-7.0, filter through a 0.22 μm microporous membrane, remove the primary filtrate, and collect the secondary filtrate as the sample solution.
[0024] Precisely measure about 0.0040 g of the dermatan sulfate standard product, add 1 g of the subsequent filtrate, and fully dissolve it as a reference solution.
[0025] Connect the ultraviolet detector to the chromatographic column, adjust its detection wavelength to 205nm, and take 10 μl of the sample solution and reference solution for injection and detection.
[0026] Calculate the content of dermatan sulfate in th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com