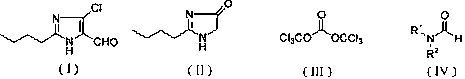
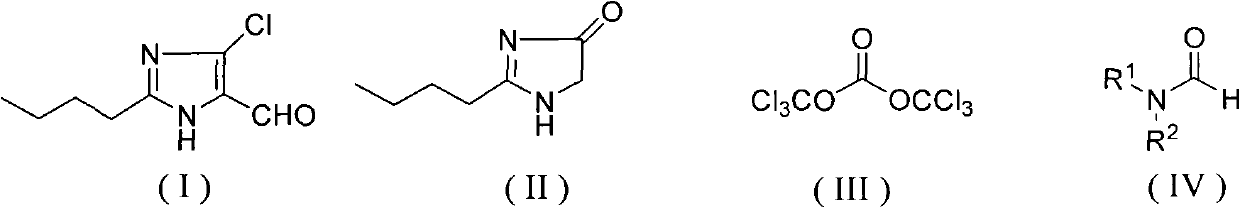
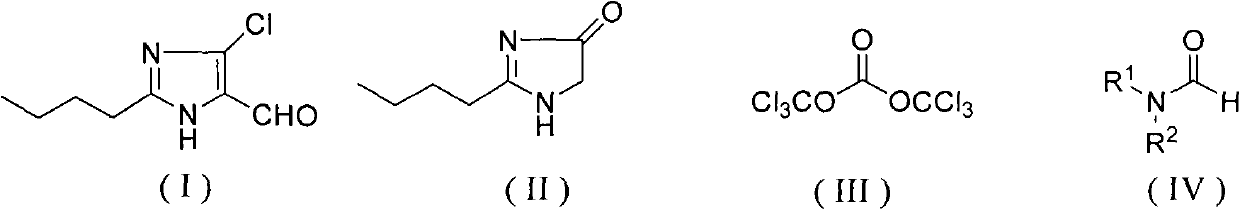
Method for synthesizing 2-normal-butyl-4-chloro-5-formylimidazole
A technology of formyl imidazole and n-butyl is applied in the field of synthesis of losartan intermediate 2-n-butyl-4-chloro-5-formyl imidazole, which can solve the problems of difficult separation and purification of products, high cost of waste treatment, Harsh reaction conditions and other problems, to achieve the effect of low cost of waste treatment, easy separation and purification, and mild reaction conditions
Active Publication Date: 2010-09-22
ZHEJIANG UNIV OF TECH +1
View PDF6 Cites 4 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The above-mentioned methods all use a large amount of highly toxic phosphorus oxychloride, and a large amount of phosphorus-containing wastewater is produced in the industrial production process, resulting in high cost for the treatment of the three wastes, and there are disadvantages such as unsafe operation, difficult separation and purification of products, and harsh reaction conditions.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Login to View More
Abstract
The invention discloses a method for synthesizing 2-normal-butyl-4- chloro-5-formylimidazole shown by a formula (I). Imidazolone shown in the formula (II) reacts with bis (trichloromethyl) carbonate shown by a formula (III) and formamide shown by a formula (IV) in an organic solvent for 1-10 hours at the temperature of 20 DEG C below zero to 200 DEG C, and reaction solution is hydrolyzed to obtain 2-normal-butyl-4-chloro-5-formylimidazole. The invention has mild reaction conditions, good selectivity, no generation of phosphorus wastewater and low cost of proposal of waste water, waste gases and waste residues, and is suitable for industrial production.
Description
technical field The invention relates to a synthesis method of a losartan intermediate 2-n-butyl-4-chloro-5-formyl imidazole. Background technique 2-n-butyl-4-chloro-5-formyl imidazole is a key intermediate of losartan, a non-peptide angiotensin II receptor blocker. U.S. Patent No. 5,442,076 reports that 2-n-butyl-3,5-dihydroimidazol-4-ketone is treated with phosphorus oxide and N,N-dimethylformamide to obtain 2-n-butyl-4-chloro-5 - formyl imidazole. U.S. Patent No. 5,486,617 reported that 2-n-butyl-3,5-dihydroimidazol-4-ketone was treated with phosphorus oxychloride and N,N-dimethylformamide dimethyl acetal to obtain 2-n-butyl -4-Chloro-5-formyl imidazole. U.S. Patent No. 5,606,072 reported that 2-n-butyl-3,5-dihydroimidazol-4-ketone was first treated with chlorination reagents such as phosphorus trichloride or thionyl chloride to obtain 2-n-butyl-5-chloroimidazole, Then, in the presence of phosphorus oxychloride, react with N,N-dimethylformamide to obtain 2-n-butyl-...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07D233/68
Inventor 金灿苏为科
Owner ZHEJIANG UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com