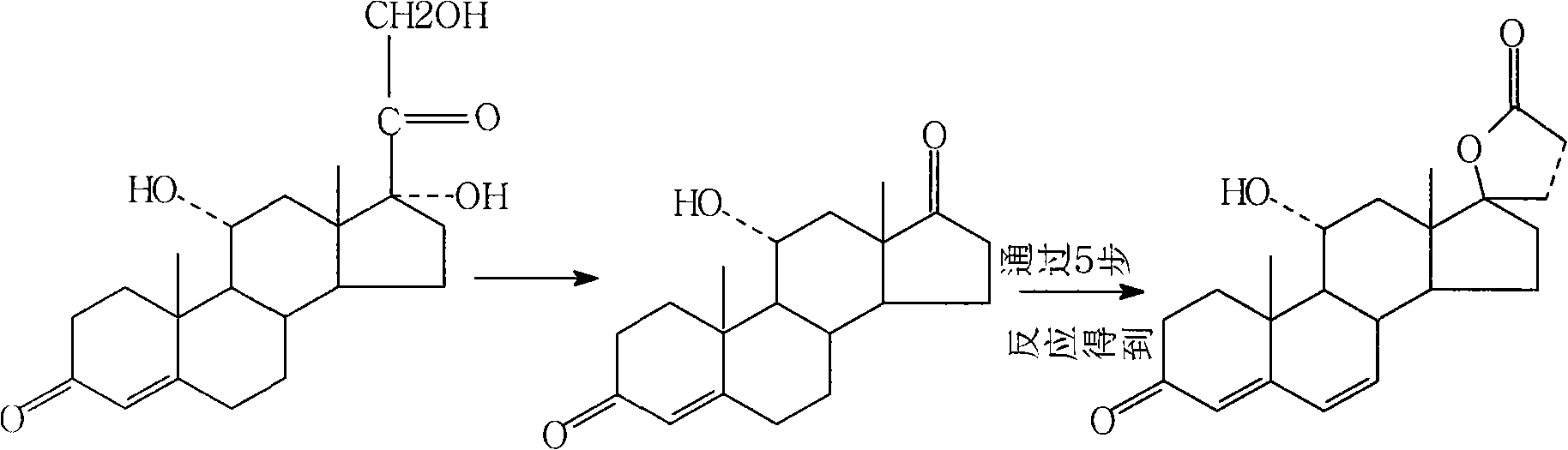
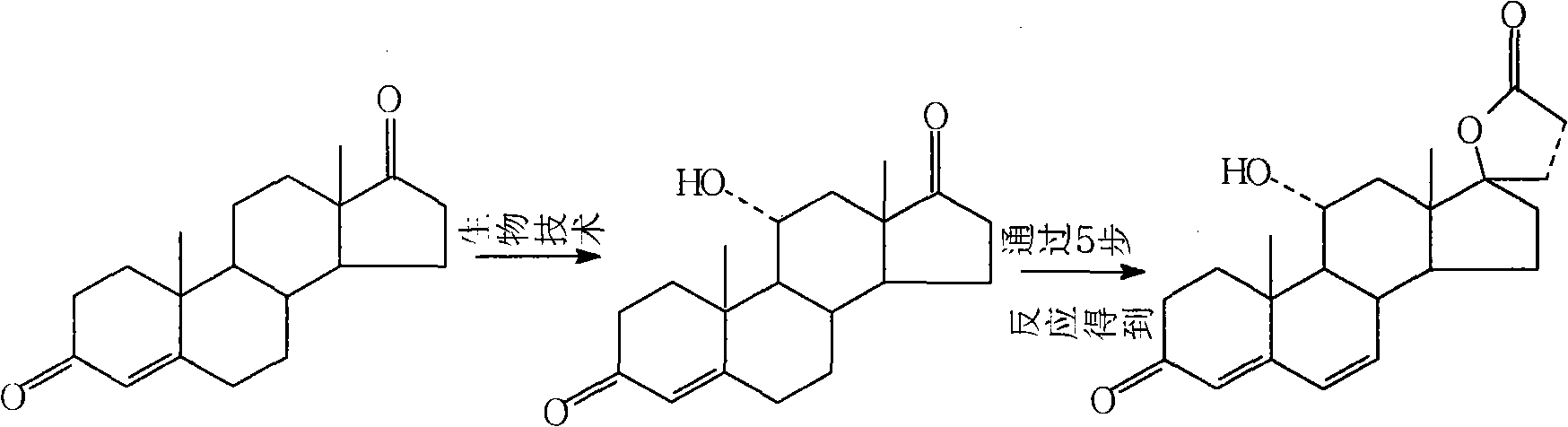
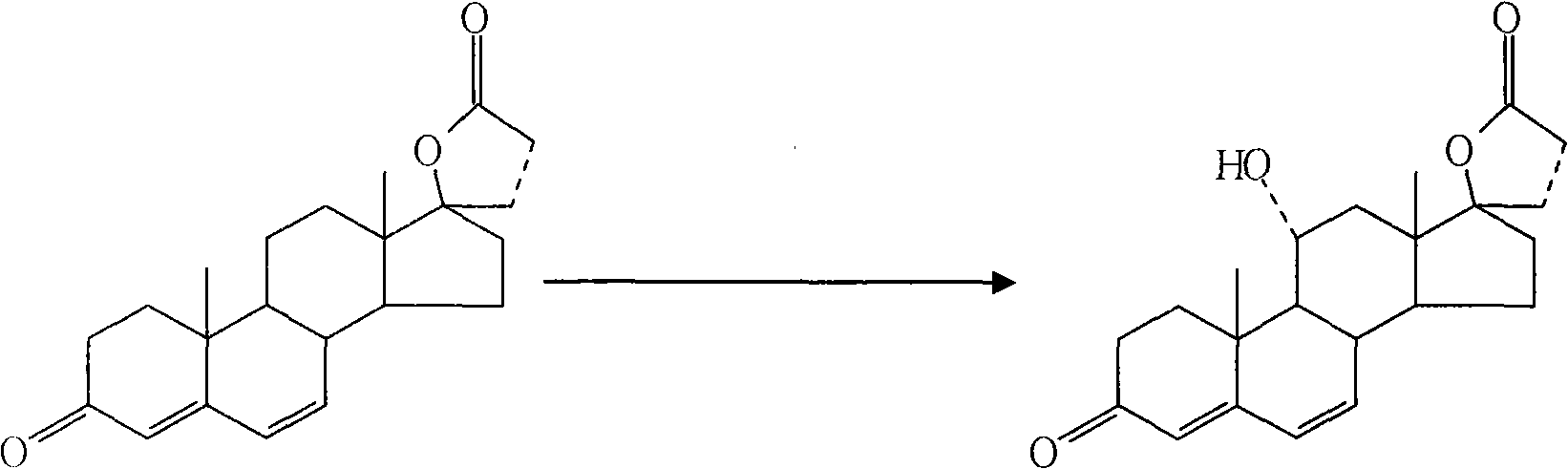
Synthetic method of 11alpha hydroxy-canrenone
A technique for the synthesis of hydroxycanrenone, which is applied in the field of biochemistry, can solve the problems of low feed concentration, poor canrenone specificity, and low yield, and achieve high feed concentration, good substrate specificity, and high product yield. The effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A method for synthesizing 11α-hydroxycanrenone. The raw materials canrenone, propylene glycol, and Tween 80 are heated and dissolved to form a substrate, which is then added to a culture medium, and then put into the culture medium for fermentation. After the fermentation is completed, the mycelium Body extraction, refined to get 11α hydroxycanrenone. Specifically include:
[0030] 1) Selection of strains: after artificial induction (15 minutes of ultraviolet radiation with a wavelength of 254nm) and natural mutation of ochratos (original strains) into excellent ochratos strains with high selectivity to the substrate canrenone Store freeze-dried tubes at low temperature.
[0031] 2), the cultivation of strains: subculture solid medium ratio: 0.07% peptone, 1% maltose, 1.5% glucose, 2.5% agar, adjust the pH to 5.2, culture conditions: 25 ° C, culture for 7 days, obtained by three generations of subculture The strains used in the production are stored in a low-temperatu...
Embodiment 2
[0036] A method for synthesizing 11α-hydroxycanrenone. The raw materials canrenone, propylene glycol, and Tween 80 are heated and dissolved to form a substrate, which is then added to a culture medium, and then put into the culture medium for fermentation. After the fermentation is completed, the mycelium Body extraction, refined to get 11α hydroxycanrenone. Specifically include:
[0037] 1. Selection of strains: Ochratos (original strain) is artificially induced (15 minutes of ultraviolet radiation with a wavelength of 254nm) and naturally mutated into an excellent strain of Ochras with high selectivity to the substrate canrenone, and then frozen Store in a dry tube at low temperature.
[0038] 2. Cultivation of strains: subculture solid medium ratio: 0.1% peptone, 2% maltose, 2% glucose, 2% agar, adjust the pH to 5.3, culture conditions: 25-30°C, culture for 9 days, take three generations of passage The strains used for production are obtained, and stored in a low-temperat...
Embodiment 3
[0043] A method for synthesizing 11α-hydroxycanrenone. The raw materials canrenone, propylene glycol, and Tween 80 are heated and dissolved to form a substrate, which is then added to a culture medium, and then put into the culture medium for fermentation. After the fermentation is completed, the mycelium Body extraction, refined to get 11α-hydroxycanrenone. Specifically include:
[0044] 1. Selection of strains: Ochratos (original strain) is artificially induced (18 minutes of ultraviolet radiation with a wavelength of 254nm) and naturally mutated into an excellent Ochras strain with high selectivity to the substrate canrenone, and then frozen Store in a dry tube at low temperature.
[0045] 2. Cultivation of strains: subculture solid medium ratio: 0.12% peptone, 1.5% maltose, 2.5% glucose, 1.8% agar, adjust the pH to 5.0, culture conditions: 25-30°C, culture for 7 days, take three generations of passage The strains used for production are obtained, and stored in a low-temp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com