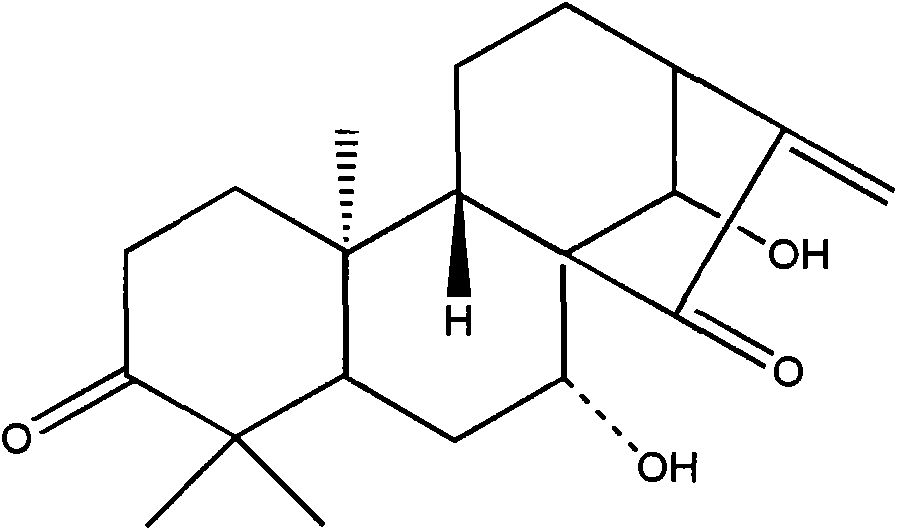
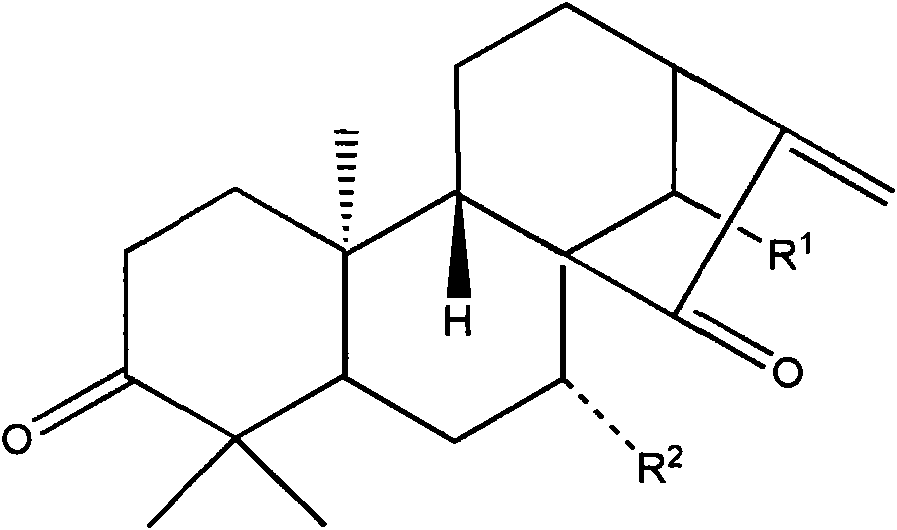
Glaucocalyxin A derivative, preparation method and application thereof
A technology of cyanine A and its derivatives, which is applied in the direction of drug combinations, pharmaceutical formulas, medical preparations containing active ingredients, etc., and can solve the problems of unreported pharmacological effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] The preparation of embodiment 1 cyanine A (GLA) derivative:
[0067] The chemical reagents used in the following preparation process are analytically pure unless otherwise specified.
[0068] Using fragrant tea herbs (aerial parts) as a raw material, purifying cyanine A (GLA), and modifying its structure with a polypeptide cNGQGEQc, to prepare a method for preparing cyanine A (GLA) derivatives, specifically comprising the following steps:
[0069] Step 1: Crush
[0070] Take fragrant tea and vegetable medicinal materials (aerial parts) and pulverize to 20 mesh to 50 mesh.
[0071] Step 2: Extract
[0072] Step 2.1: Mix the pulverized product obtained in step 1 with 95% ethanol (A.R.) at a volume ratio of 1:6 to 1:10, heat, reflux at 80°C to 90°C for 1 to 2 hours, extract and filter, An extract and a residue are obtained.
[0073] Step 2.2: Mix the residue obtained in step 2.1 with 95% ethanol (A.R.) at a volume ratio of 1:6 to 1:10, heat, reflux at 80°C to 90°C for ...
Embodiment 2
[0107] The preparation of embodiment two cyanine A (GLA) derivatives:
[0108] Adopt the following technical parameters to improve the preparation method of embodiment one:
[0109] In the step 2.1, the pulverized product is mixed with 95% ethanol (A.R.) in a volume ratio of 1:6.5, heated, refluxed at 82° C. for 1.8 hours, and then extracted and filtered.
[0110] In the step 2.2, the residue is mixed with 95% ethanol (A.R.) at a volume ratio of 1:9.5, heated, refluxed at 88° C. for 1.2 hours, extracted and filtered, and this step is repeated once.
[0111] In the step 3.1, the extract is heated and concentrated under reduced pressure at a temperature of 57°C.
[0112] In the step 3.2, the primary concentrate and water were mixed in a volume ratio of 1:8.5, stirred at room temperature and then left to stand for 11 hours before the supernatant and solid were separated.
[0113] In the step 3.3, the solid was mixed with ethyl acetate (A.R.) at a volume ratio of 1:5.5, stirred ...
Embodiment 3
[0130] Preparation of embodiment three cyanine calyxin (GLA) derivatives:
[0131] Adopt the following technical parameters to improve the preparation method of embodiment one:
[0132] In the step 2.1, the pulverized product is mixed with 95% ethanol (A.R.) at a volume ratio of 1:7.5, heated, refluxed at 84° C. for 1.6 hours, and then extracted and filtered.
[0133] In the step 2.2, the residue is mixed with 95% ethanol (A.R.) at a volume ratio of 1:8.5, heated, refluxed at 86° C. for 1.4 hours, extracted and filtered, and this step is repeated once.
[0134] In the step 3.1, the extract is heated and concentrated under reduced pressure at a temperature of 59°C.
[0135] In the step 3.2, the primary concentrate and water were mixed at a volume ratio of 1:8.5, stirred at room temperature and then allowed to stand for 10 hours to separate the supernatant and solid.
[0136] In the step 3.3, the solid was mixed with ethyl acetate (A.R.) at a volume ratio of 1:5.5, stirred and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com