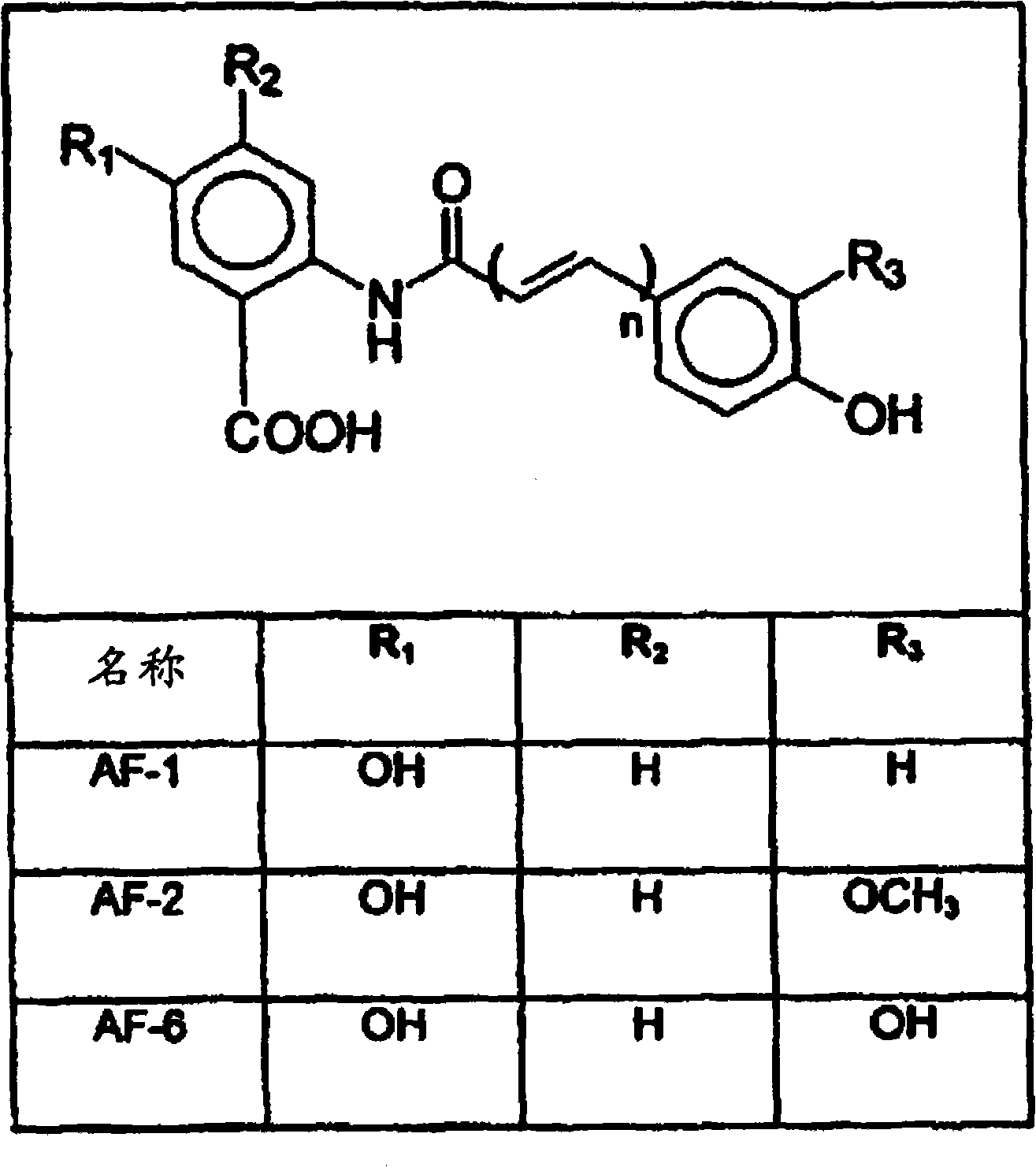
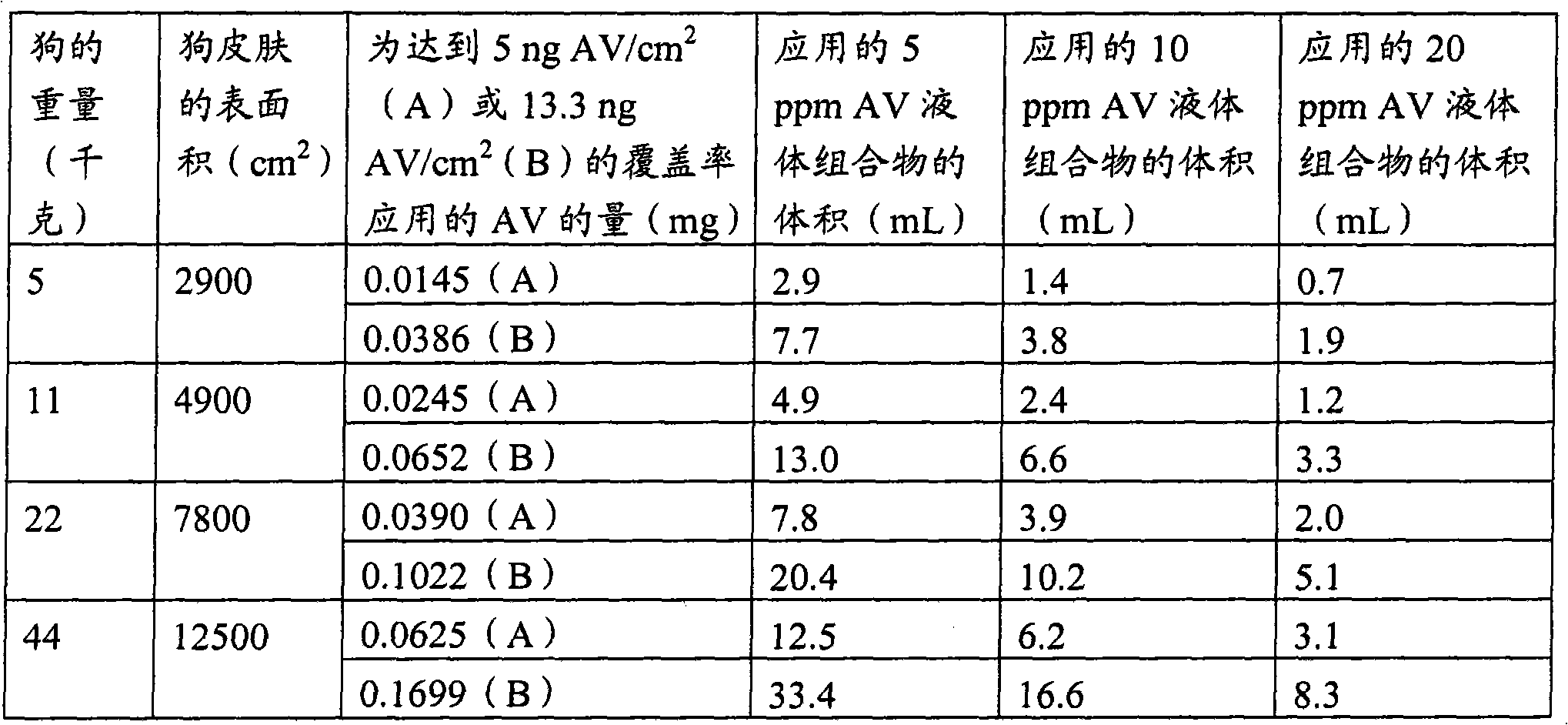
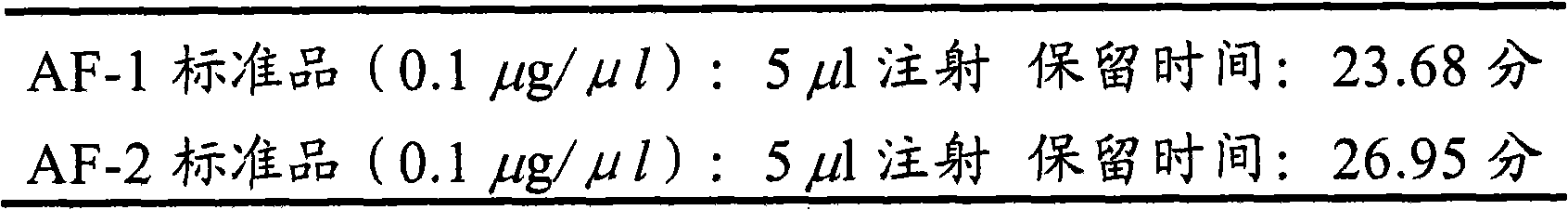Avenanthramide-containing compositions
A technology of oat anthramid and composition, which is applied in the direction of drug combinations, medical preparations containing active ingredients, plant raw materials, etc., and can solve the problems of not providing anti-irritation properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0100] Example 12 demonstrates beta(1) prepared according to the methods described in U.S. Patent Application Nos. 10 / 554,288 and 10 / 554,290 (the disclosures of which are incorporated herein by reference) and applied as a topical composition to the surface of skin slices -3) β(1-4) glucan can obviously enter the stratum corneum, epidermis, dermis and subcutaneous tissue layer of the skin. These results demonstrate that the parasiticide encapsulated by β(1-3)β(1-4) glucan isolated according to this method can also efficiently transfer to the dermis and subcutaneous tissue layers of the subject's skin. Therefore, the therapeutic / parasiticidal pharmaceutical compositions of the present invention comprising β(1-3)β(1-4) glucan are advantageous in that they can easily transport ecto- and / or endoparasiticides to The dermis and subcutaneous tissue layers of animal skin.
[0101] The pharmaceutical compositions of the present invention may also contain various known and conventional ...
Embodiment 1
[0135] Preparation method of oat extract
[0136] For each method, 2 or 3 replicates were performed and analyzed.
[0137] Method: Hulled oat kernels (Hinoat variety) were ground through a Willey Mill to pass a 10 mesh screen. To a stirred 50% (v / v) aqueous ethanol solution at 40°C was added a 1:4 (w / v) oatmeal:solvent mix ratio of oatmeal. The resulting mixture was stirred for 30 minutes and then cooled to room temperature. The mixture was then centrifuged at 2830 g for 7 minutes and the supernatant was aspirated. The pellet was resuspended in fresh solvent and centrifuged again. The supernatant was aspirated and the pellet was resuspended a third time in fresh solvent. All supernatants were combined and filtered through coarse sintered glass filters.
[0138] In order to show the difference between the method (process) for preparing the oat extract according to the present invention, which includes the step of adjusting the pH of the extract to <4.0, and the method with...
Embodiment 2
[0165] Scale-up test of oat extraction method
[0166] Method: The hulled oat kernels (AC Ernie variety) were ground through a Willey Mill to pass through a 10 mesh screen. Oatmeal (1.5 kg) was added to a stirred 50% (v / v) aqueous ethanol solution (6000 ml) at 40°C. The resulting mixture was stirred for 30 minutes and then cooled to room temperature. The mixture was then centrifuged at 2830 g for 7 minutes and the supernatant was aspirated. The pellet was resuspended in fresh solvent (3000ml) and centrifuged again. The supernatant was aspirated and the pellet was resuspended a third time in fresh solvent. All supernatants were pooled and filtered through coarse sintered glass filters. The pH of the extract was adjusted to 3.5 with hydrochloric acid (1 M) and ethanol (~1%) was added to clarify the solution. The pale yellow extract was passed through a 0.45 μm filter (Gelman; Supor DCF), made up to 12000 ml, and then ultrafiltered.
[0167] Using Pall Corporation CENTRASET...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com