Method for preparing phloroglucinol injection
A technology of phloroglucinol injection and phloroglucinol, which is applied in the fields of pharmaceutical formula, drug delivery, digestive system, etc., can solve the problems such as the decrease of trimethylphloroglucinol content, and achieve the assurance level of sterility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
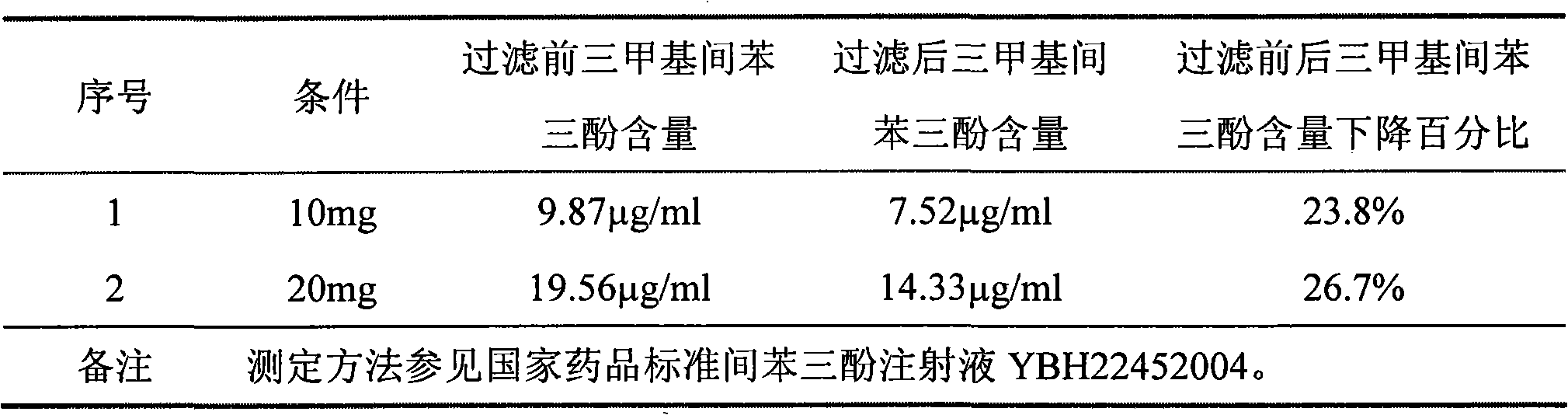
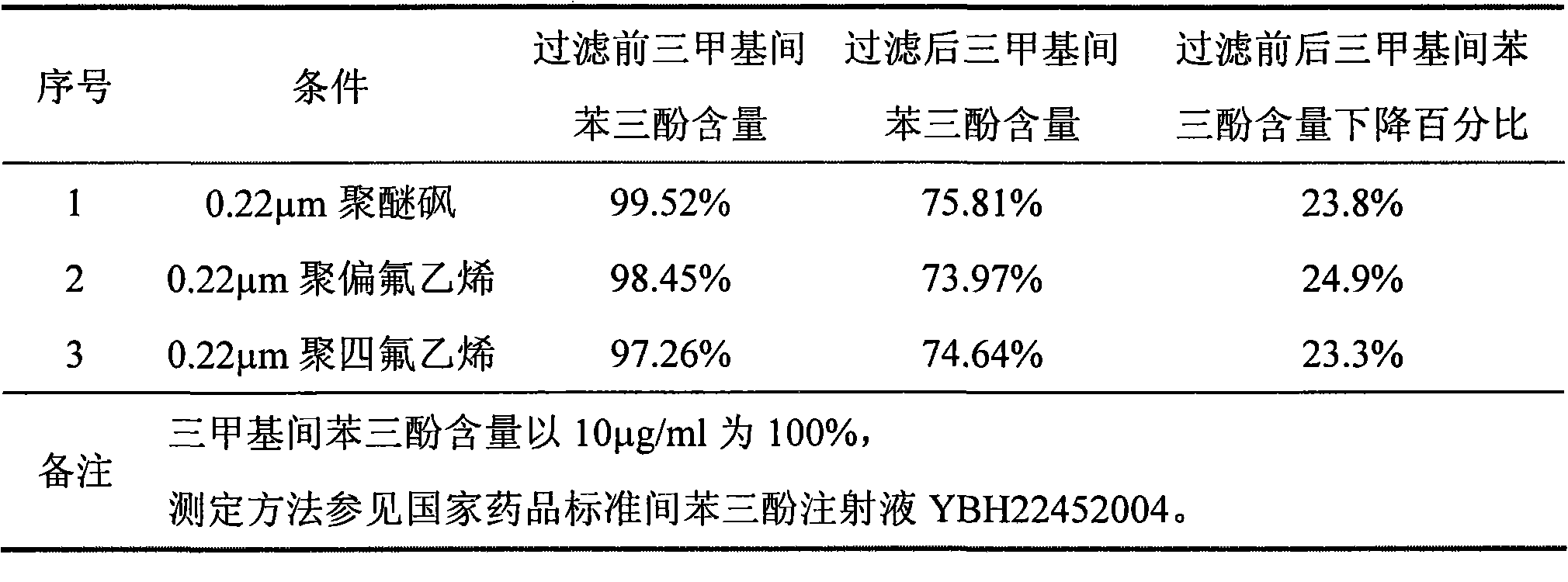
[0019] Example 1 Study on the decrease of trimethylphloroglucinol content during the filtration process of phloroglucinol injection 1.1 Whether the decrease of trimethylphloroglucinol content during the filtration process is related to its low solubility in water.
[0020] The comparative experiment is as follows: Weigh 7 g of sodium chloride, 1 g of sodium bisulfite, 0.35 g of citric acid, and 1.025 g of disodium hydrogen phosphate dodecahydrate, add a small amount of water for injection to dissolve; add 10 g of phloroglucinol, and make up water for injection When the volume of the solution is 1000mL, stir to dissolve to obtain solution A; prepare two parts of solution A, dissolve 10 mg and 20 mg of trimethylphloroglucinol with 2 ml of ethanol respectively, add to solution A, stir evenly, and take samples to check the trimethyl phloroglucinol Phloroglucinol content; at the same time, 0.22 μm polyethersulfone filter membrane sterilization filtration while hot, check the trimeth...
Embodiment 2
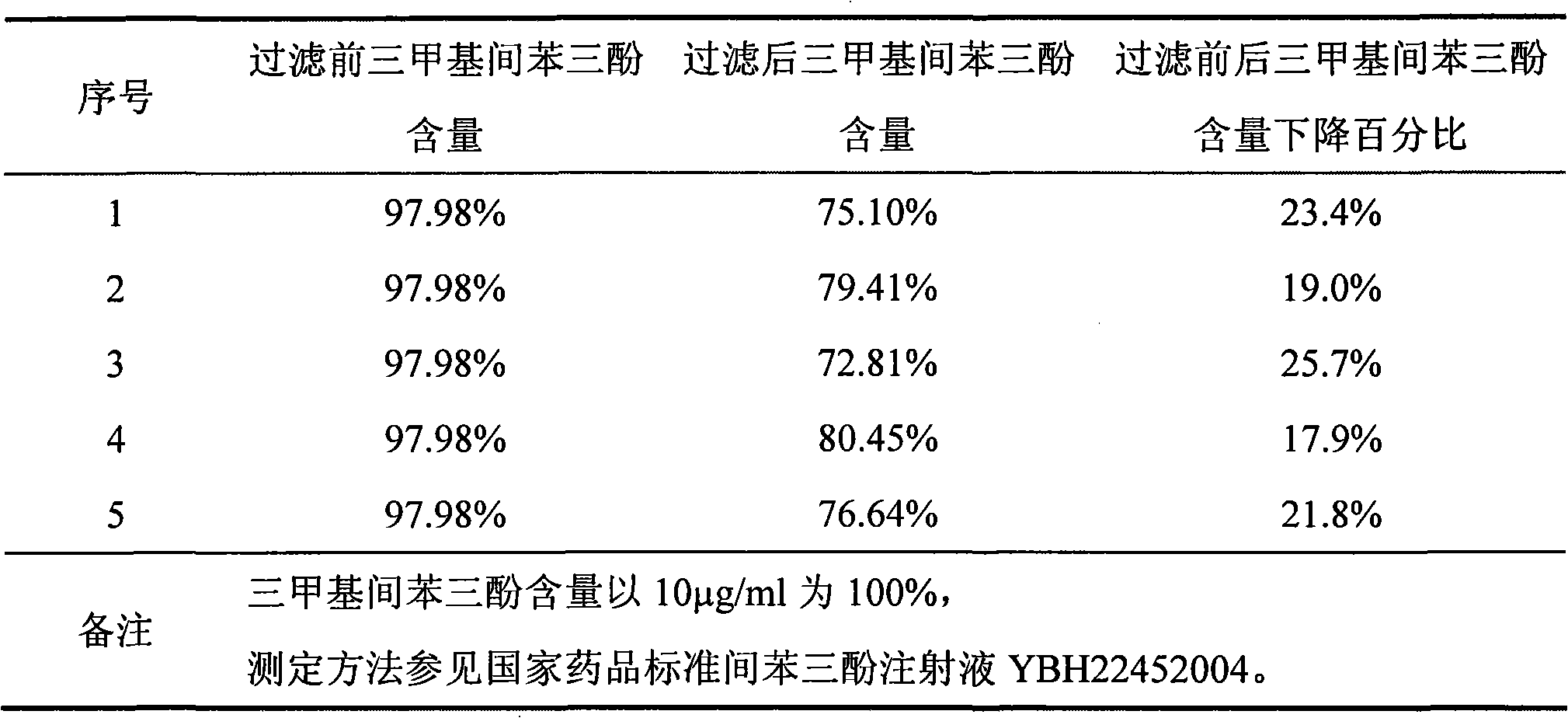
[0042] Weigh 7g of sodium chloride, 1g of sodium bisulfite, 0.35g of citric acid and 1.025g of disodium hydrogen phosphate dodecahydrate, add a small amount of water for injection to dissolve; add 10g of phloroglucinol, and make up water for injection until the volume of the solution is 1000mL, stir to make it dissolve, add 1g of activated carbon and stir evenly, decarbonize with 1μm filter membrane to obtain the filtrate; dissolve 10mg of trimethylphloroglucinol with 20ml of 75% ethanol aqueous solution, filter through a 0.22μm filter membrane, add Add to the above filtrate, stir evenly, to obtain the intermediate product solution, check the phloroglucinol content, trimethylphloroglucinol content and pH of the intermediate product solution; reduce the temperature of the intermediate product solution to 5 ° C, filter 0.45 μm polyethersulfone Membrane sterilization filtration, check filtrate (finished solution) phloroglucinol content, trimethyl phloroglucinol content and pH. Af...
Embodiment 3
[0046] Weigh 7g of sodium chloride, 1g of sodium bisulfite, 0.35g of citric acid and 1.025g of disodium hydrogen phosphate dodecahydrate, add a small amount of water for injection to dissolve; add 10g of phloroglucinol, and make up water for injection until the volume of the solution is 1000mL, stir to dissolve, add 1g of activated carbon and stir evenly, decarbonize with 1μm filter membrane to obtain filtrate; dissolve 10mg of trimethylphloroglucinol with 10ml of ethanol, filter through 0.22μm filter membrane, add to the above filtrate, stir Evenly, get the intermediate product solution, check the intermediate product solution phloroglucinol content, trimethylphloroglucinol content and pH, reduce the temperature of the filtered solution to 10 ° C, 0.22 μm polyethersulfone sterile filtration, check the filtrate (finished product solution ) phloroglucinol content, trimethylphloroglucinol content and pH. After passing the test, potting and steam sterilization at 121°C for 20 min...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com