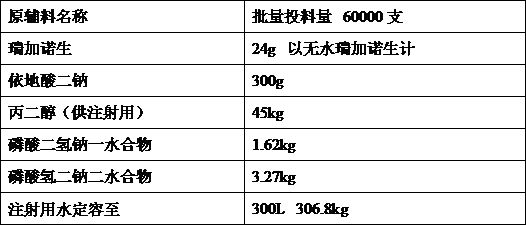
Preparation method of regadenoson injection
A technology of Reganoson and injection, which is applied in the field of preparation of Reganoson injection, and can solve problems such as the increase of insoluble particles and the impact on product safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1 Feeding sequence
[0019] Investigate the impact of feeding order on product quality. In order to minimize the impact of metal ions in the production process, edetate disodium should be added first, and propylene glycol is a co-solvent. Therefore, two feeding orders are preliminarily drawn up. Other process parameters are Consistent, only change the feeding sequence of raw and auxiliary materials.
[0020]
[0021] From the experimental data, it can be seen that the two feeding processes have no obvious impact on the quality of intermediate products. Considering the production needs, the one with the shortest liquid mixing time is selected, and process 1 is selected as the liquid feeding process.
Embodiment 2
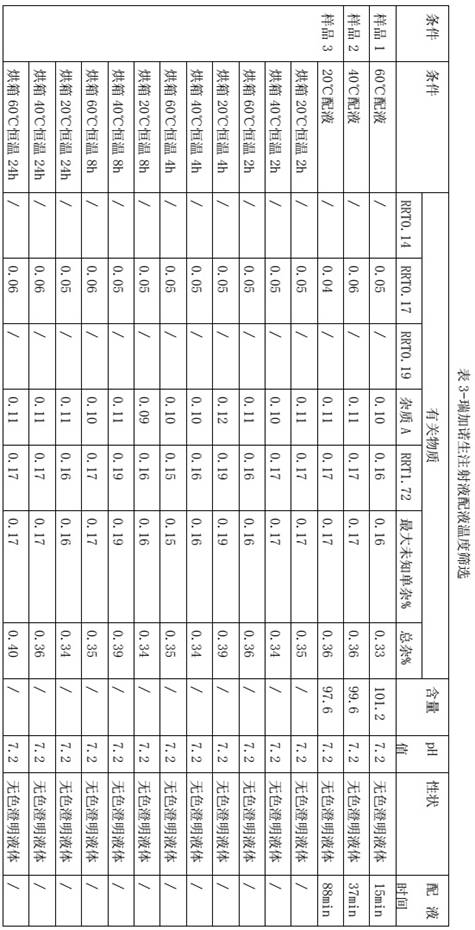
[0022] The screening of embodiment 2 dosing temperature
[0023] The batching temperature affects the dissolution rate of reganosen and the stability of the intermediate solution. Considering that the high temperature of the dosing solution may increase the risk of impurity growth, combined with the actual production needs of the pharmaceutical factory, it is set to 20°C, 40°C, Screening at 60°C dosing temperature, and investigating the stability of the intermediate solution at 20°C, 40°C, and 60°C for 2 hours, 4 hours, 8 hours, and 24 hours, so as to screen the dosing temperature, other processes The parameters are all the same, only the dosing temperature is changed.
[0024]
[0025] The higher the temperature, the shorter the dosing time. Under the conditions of 20°C and 40°C in the oven for 24 hours, the impurities of the self-developed product have no growth trend, and there is no increase trend in the oven at 60°C for 8 hours. In the oven at 60°C for 24 hours, the se...
Embodiment 3
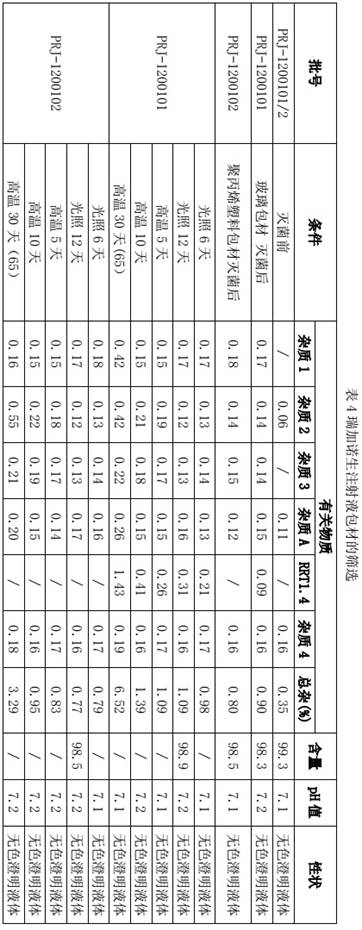
[0026] Embodiment 3: Screening of packaging materials
[0027] Phosphate buffer solution is used as a pH regulator in the prescription of Reganoson Injection. Phosphorus in the phosphate buffer solution interacts with the solution in the glass ampoule to cause precipitation, which may lead to the increase of insoluble particles in the injection solution and affect the product. For safety, the present invention uses BFS plastic packaging, and uses glass packaging materials and plastic packaging materials to package Reganoson Injection, and investigates the impact of packaging materials on Reganoson Injection.
[0028]
[0029] From the test results, the use of glass packaging materials to package Regganosan Injection produces a new impurity RRT1.4, while the use of plastic packaging materials does not produce new impurities, and the cost is lower, which is conducive to industrial production.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com