Synthesis of hydrofluoroalkanols and hydrofluoroalkenes
A technology for hydrofluoroalkanols and hydrofluoroalkenes, which can be applied to compounds of Group 2/12 elements of the periodic table, organic compounds of Group 2/12 without C-metal bonds, preparation of halogenated hydrocarbons, etc., and can solve the limitation And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1 shows the preparation of 2-chloro-3,3,3-trifluoropropanol from 1,1,1,2-tetrafluoro-2,2-dichloroethane.
[0058] in N 2 Add 32.8g (0.5mol) of active zinc powder, 12g (0.5mol) of paraformaldehyde and 180mL of anhydrous DMF into a 400mL Hastelloy C shaking tube. The tube was cooled to -15°C, and 64.4 g (0.2 mol) of 1,1-dichlorotetrafluoroethane was added. The reaction mixture was then stirred at 50°C for 6 hours. The results of gas chromatographic analysis of the reactions are summarized in Table 1. After the reaction mixture was cooled to room temperature, it was poured into a mixture of 200 mL of ice, 10% aqueous HCl and 200 mL of diethyl ether with stirring. After stirring for another 30 min, the organic layer was separated and washed with 100 mL of 2% aqueous HCl followed by 100 mL of brine. Using MgSO 4 After it was dried, the ether was removed in vacuo to obtain 13.36 g of product (8% yield).
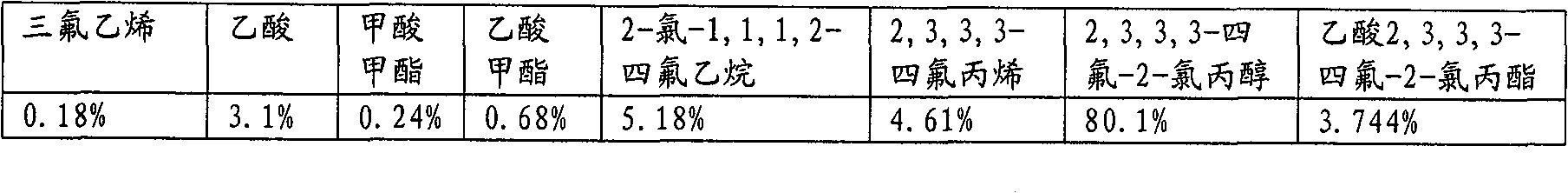
[0059] Table 1
[0060] components
Embodiment 2
[0062] Example 2 shows the conversion of 2-chloro-2,3,3,3-tetrafluoropropanol to 2,3,3,3-tetrafluoro-1-propene.
[0063] in N 2 Add 26g (0.4mol) active zinc powder, 33.3g (0.2mol) 2-chloro-2,3,3,3-tetrafluoropropanol, 30.6g (0.3mol) ethyl alcohol to the 400mL Hastelloy C shaking tube anhydride and 180 mL of anhydrous DMF. The reaction mixture was then stirred at 50°C for 6 hours. After the reaction mixture was cooled to room temperature, the product was collected in a cold trap frozen with dry ice to obtain 18.1 g of 2,3,3,3-tetrafluoropropene.
Embodiment 3
[0065] Example 3 shows the synthesis of 2,3,3,3-tetrafluoro-1-propene from 1,1,1,2-tetrafluoro-2,2-dichloroethane.
[0066] in N 2 Add 20 g (0.315 mol) of active zinc powder, 7.5 g (0.25 mol) of paraformaldehyde and 130 mL of anhydrous DMF into a 400 mL Hastelloy C shaking tube. The tube was cooled to -15°C, and 43 g (0.25 mol) of 1,1-dichlorotetrafluoroethane was added. The reaction mixture was then stirred at 60°C for 6 hours. After the reaction mixture was cooled to room temperature, 30 g (0.46 mol) of active zinc powder and 50 g (0.5 mol) of acetic anhydride were added to the reactor. The reaction mixture was stirred at 50 °C for 6 hours, then cooled to room temperature. The gas and liquid phases were analyzed by GC-MS. The results are summarized in Table 2.
[0067] Table 2
[0068] Component (liquid phase)
[0069] Components (gas phase)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com