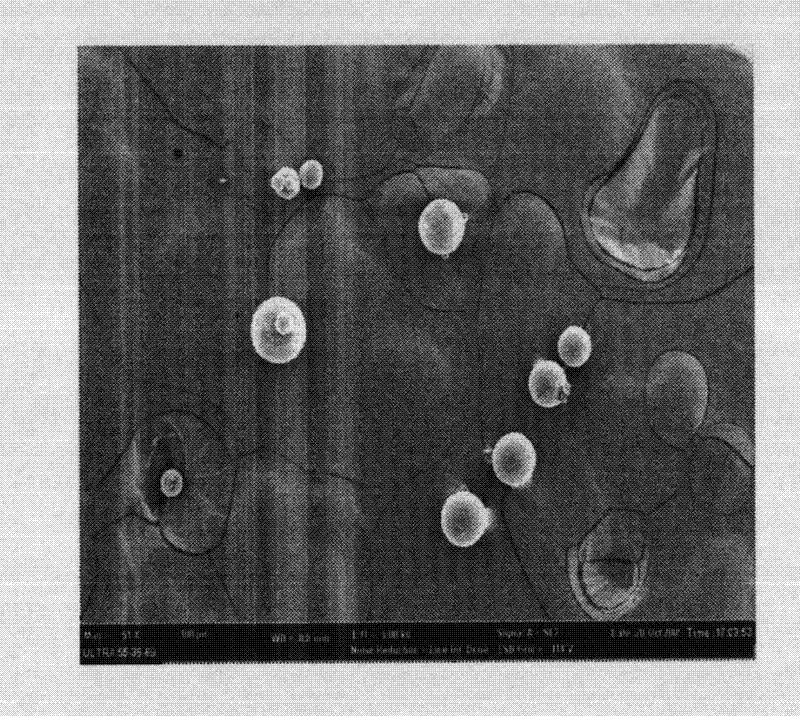
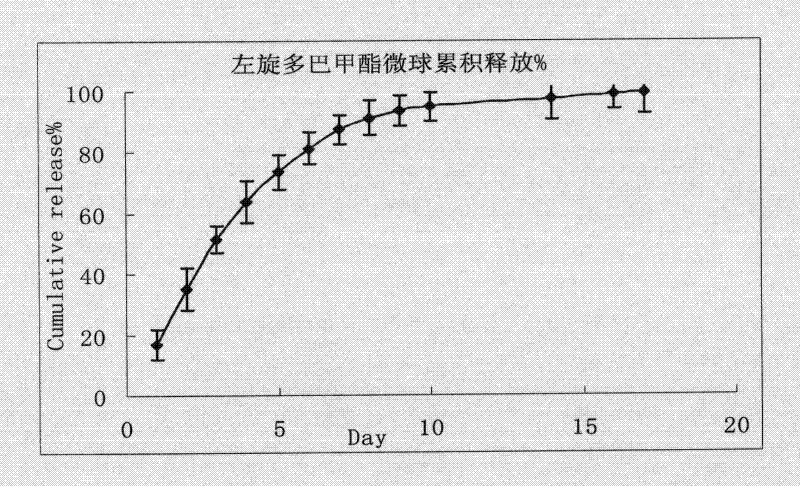
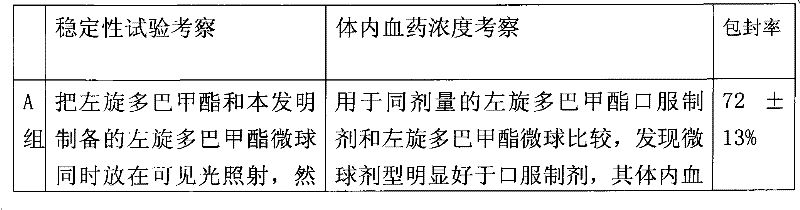
Levodopa methyl ester slow-release microsphere composition and preparation method thereof
A technology of levodopa methyl ester and sustained-release microspheres, which is applied in the direction of drug combinations, pharmaceutical formulas, and medical preparations of non-active ingredients, and can solve the problems of not preparing levodopa methyl ester sustained-release microspheres, etc., to achieve Good redispersibility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] ①Preparation of levodopa methyl ester solution
[0030] a) 100mg levodopa methyl ester is prepared into an aqueous solution with a concentration of 2.5% by weight;
[0031] ②Preparation of levodopa methyl ester sustained-release microsphere composition
[0032] (a) Mix 37.5 mg of polylactic acid (PLA, molecular weight 90,000-140,000) into an organic solution of 15% dichloromethane by weight and 0.5 mL of the above-mentioned ① levodopa methyl ester solution Stir, vortex or sonicate for 1-5 minutes to form a uniform suspension, that is, a water-in-oil (W / O) emulsion; the theoretical percentage of levodopa methyl ester prepared into sustained-release microspheres is 25%.
[0033] (b) adding the emulsion obtained in step (a) to 10 mL of an aqueous solution of 5% sodium chloride and 1% polyethylene glycol (the molecular weight of PVA is 146,000-186,000, alcoholysis degree 98-99%) with a weight percent concentration and Stir, vortex or sonicate for 0.1-5 minutes to form dou...
Embodiment 2
[0041] ①Preparation of levodopa methyl ester solution
[0042] a) 100mg levodopa methyl ester is prepared into an aqueous solution with a concentration of 2.5% by weight;
[0043] ②Preparation of levodopa methyl ester sustained-release microsphere composition
[0044] (a) the polylactic acid (PLA, molecular weight is 90,000-140,000) that weighs 295mg is mixed with the organic solution that is the dichloromethane of 15% concentration by weight and takes 0.2mL above-mentioned ① levodopa methyl ester solution and mixes and Stir, vortex or sonicate for 1-5 minutes to form a uniform suspension, that is, a water-in-oil (W / O) emulsion; the theoretical percentage of levodopa methyl ester to be prepared is 1% sustained-release microspheres.
[0045] (b) adding the emulsion obtained in step (a) to 10 mL of an aqueous solution of 5% sodium chloride and 1% polyethylene glycol (the molecular weight of PVA is 146,000-186,000, alcoholysis degree 98-99%) with a weight percent concentration a...
Embodiment 3
[0053] ①Preparation of levodopa methyl ester solution
[0054] a) 100mg of levodopa methyl ester is prepared to have a concentration of 2.5% by weight;
[0055] ②Preparation of levodopa methyl ester sustained-release microsphere composition
[0056] a) Measure 0.2mL, 0.5mL or 1mL of the levodopa methyl ester solution obtained in ① and weigh 595mg of polylactic acid (PLA molecular weight is 6000), 37.5mg of polylactic acid (PLGA molecular weight is 250,000) or 25mg Polylactic acid (PLGA molecular weight is 500,000) and prepared into organic solutions of dichloromethane with a concentration of 30%, 15% or 5% by weight; 5% and 1 mL of the above-mentioned levodopa methyl ester solutions are mixed one by one correspondingly and stirred, vortexed or sonicated for 1-5 minutes to form a uniform suspension, that is, a water-in-oil (W / O) emulsion; The percentage content of levodopa methyl ester is 1% or 50% slow-release microspheres.
[0057] b) Add the emulsion obtained in step (a) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com