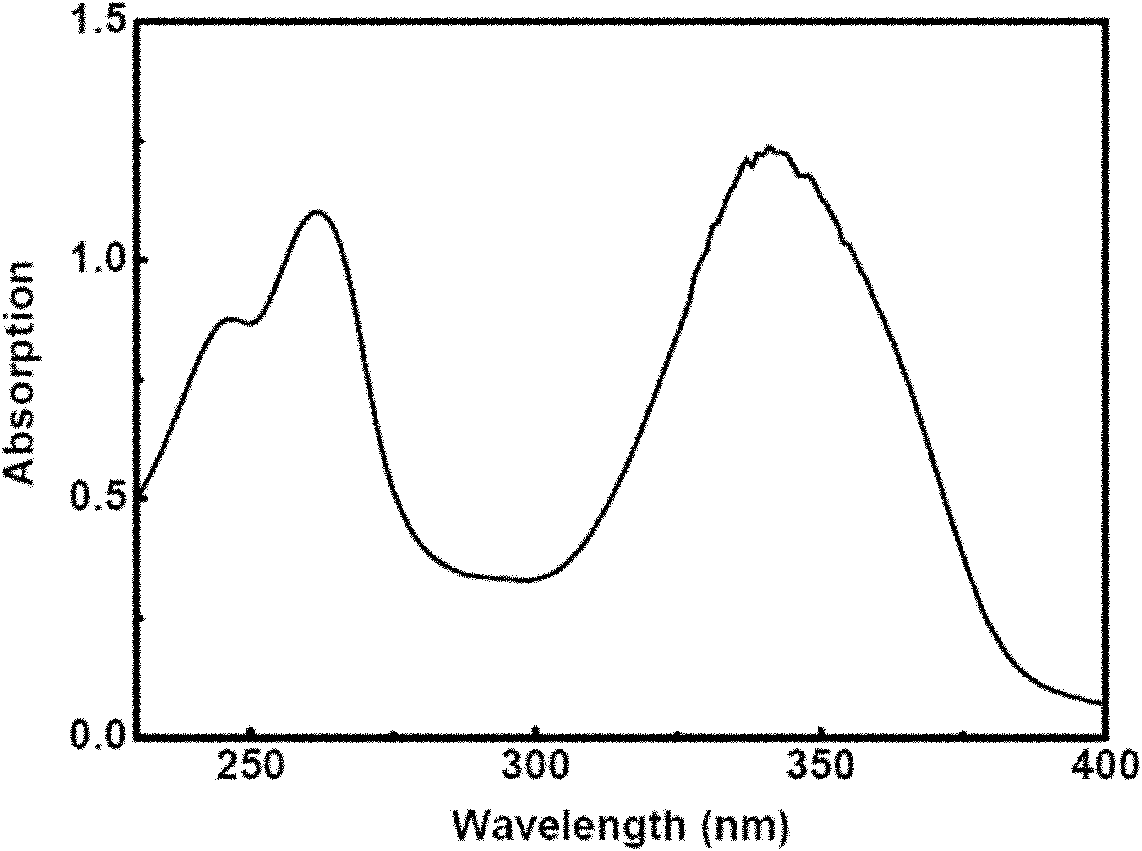
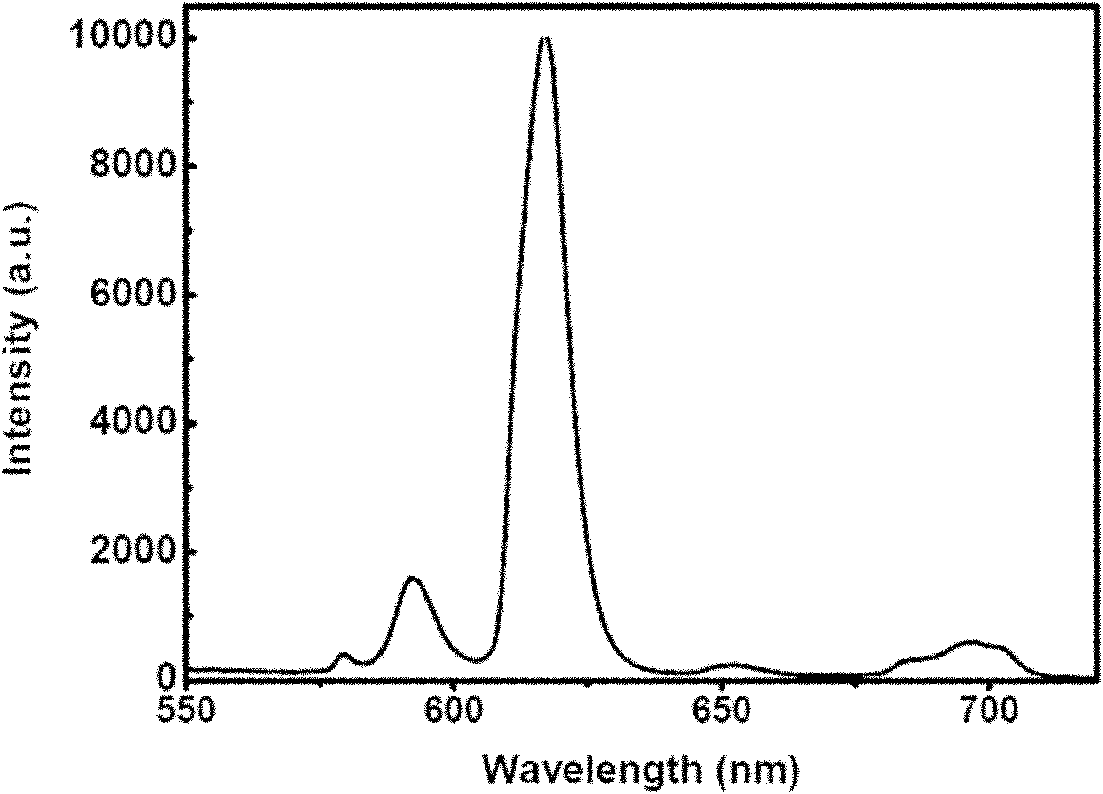
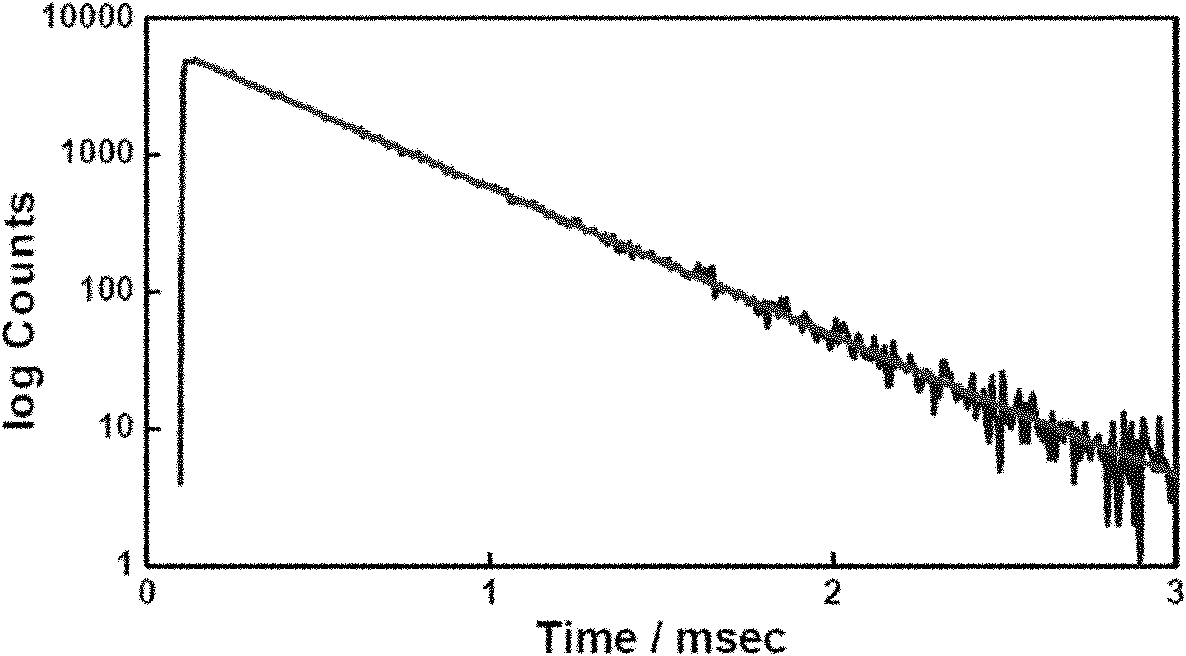
Rare-earth compound olefin monomer and preparation method and application thereof
A technology of olefinic monomers and rare earths, which is applied in the field of rare earth complexes of olefinic monomers, can solve the problems of difficulty in obtaining rare earth polymer light-emitting materials, reduction in the number of "antenna" groups, and reduction in luminous efficiency, and achieve high internal energy Effect of conversion, high luminous efficiency, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Synthesis of rare earth europium-thiophenoyltrifluoroacetone-5-allyloxymethyl-8-hydroxyquinoline complex ethylenic monomer and its copolymer:
[0046] Take 58.1g (1.0mol) allyl alcohol, add 9.65g (0.05mol) 5-chloromethyl-8-hydroxyquinoline, 2.0g (0.05mol) sodium hydroxide, 0.11g (0.001mol) hydroquinone , temperature controlled at 40°C and stirred, reacted for 48h, added 90g (5mol) of water at the end of the reaction, added dropwise an appropriate amount of dilute ammonia water with a mass concentration of 1% to neutral, obtained a white precipitate, and recrystallized petroleum ether 3 times to obtain 8- Hydroxyquinoline ligand ethylenic monomer 8.2g, yield 76.3%.
[0047] Dissolve 0.4412g (1.337mmol) europium isopropanol in 25ml of toluene, add 0.59g (2.674mmol) thienoyltrifluoroacetone, blow argon, stir and react at room temperature for 2 days, add 0.323g (1.5mmol) containing 8 - the ethylenic monomer of the hydroxyquinoline ligand, stirred and reacted at room temper...
Embodiment 2
[0050] Synthesis of rare earth samarium-thienoyltrifluoroacetone-5-(4-vinylbenzyloxymethyl)-8-hydroxyquinoline complex ethylenic monomer and its copolymer:
[0051] Take 15.2g (0.1mol) p-vinylbenzyl chloride, add 0.875g (5mmol) 5-hydroxymethyl-8-hydroxyquinoline, 0.41g (5mmol) sodium acetate, 0.1g (1.0mmol) benzoquinone, temperature control Stir at 60°C, react for 24 hours, add 72g (4mol) of water after the reaction is completed, add dropwise an appropriate amount of dilute ammonia water with a mass concentration of 5% until neutral, a white precipitate is obtained, and petroleum ether is recrystallized 3 times to obtain 8-hydroxyquinoline Ligand ethylenic monomer was 1.2 g, and the yield was 82.4%.
[0052] Take 0.406g (2.0mmol) samarium hydroxide, 0.888g (4.0mmol) thienoyl trifluoroacetone dispersed in 20ml propanol, argon flow, stirring reaction at room temperature for 3 days, add 0.73g (2.5mmol) containing 8- The ethylenic monomer of the hydroxyquinoline ligand was stirre...
Embodiment 3
[0055] Synthesis of rare earth dysprosium-thienoyltrifluoroacetone-5-acryloyloxyethoxymethyl-8-hydroxyquinoline complex ethylenic monomer and its copolymer:
[0056] Take 20g (0.172mol) hydroxyethyl acrylate, add 4.2g (0.0217mol) 5-chloromethyl-8-hydroxyquinoline, 1.78g (0.0217mol) sodium acetate, 0.02g (0.182mmol) hydroquinone, The temperature was controlled at 90°C and stirred, and reacted for 7 hours. After the reaction, 25g (1.39mol) of water was added, and an appropriate amount of dilute ammonia water with a mass concentration of 3% was added dropwise to neutrality to obtain a white precipitate. Petroleum ether was recrystallized 3 times to obtain 8- Hydroxyquinoline ligand ethylenic monomer 5.1g, yield 86.1%.
[0057] Take 0.538g (2.0mmol) of dysprosium chloride, 0.888g (4.0mmol) of thienoyl trifluoroacetone dissolved in 30ml of isopropanol, 0.092g (4.0mmol) of sodium metal, argon, stirring reaction at room temperature for 2 days, Add 0.546g (2.0mmol) vinyl monomer cont...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com