Ornithine aspartate injection and preparation method thereof
A technology of ornithine aspartate and injection, which is applied in the field of medicine, can solve problems such as unclear impurities, high content of main impurities, and increased risk of adverse reactions in patients, and achieve an easy-to-control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The preparation of embodiment 1 main impurity acetate
[0044] The reaction formula is as follows:
[0045]
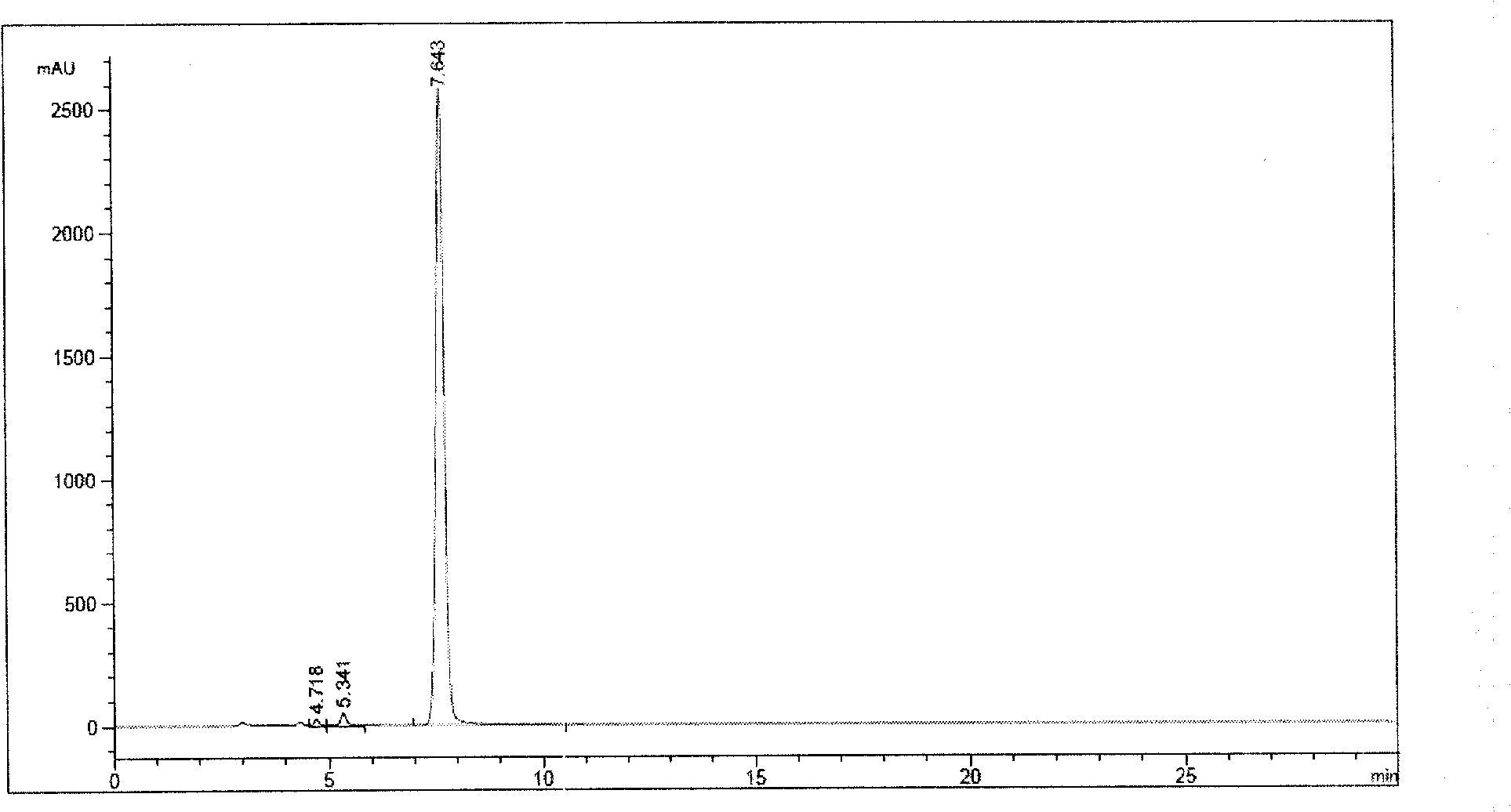
[0046] Take an appropriate amount of ornithine acetate, and add concentrated ammonia to completely dissolve it. Reflux at 100°C for 25 hours, add an appropriate amount of concentrated ammonia water every 4 hours, follow and monitor the reaction by HPLC, until the ornithine peak area normalization method in HPLC accounts for about 0.5%. At 100°C, add 0.1% activated carbon and filter. The filtrate was rotary evaporated to dryness, added methanol, cooled to 0°C, and filtered to obtain the main impurity, acetate, off-white powder. Purity greater than 99.0% by HPLC analysis, such as figure 1 shown, t R (retention time)=7.643 is the main impurity peak. The chemical characteristics of the main impurity acetate are as follows:
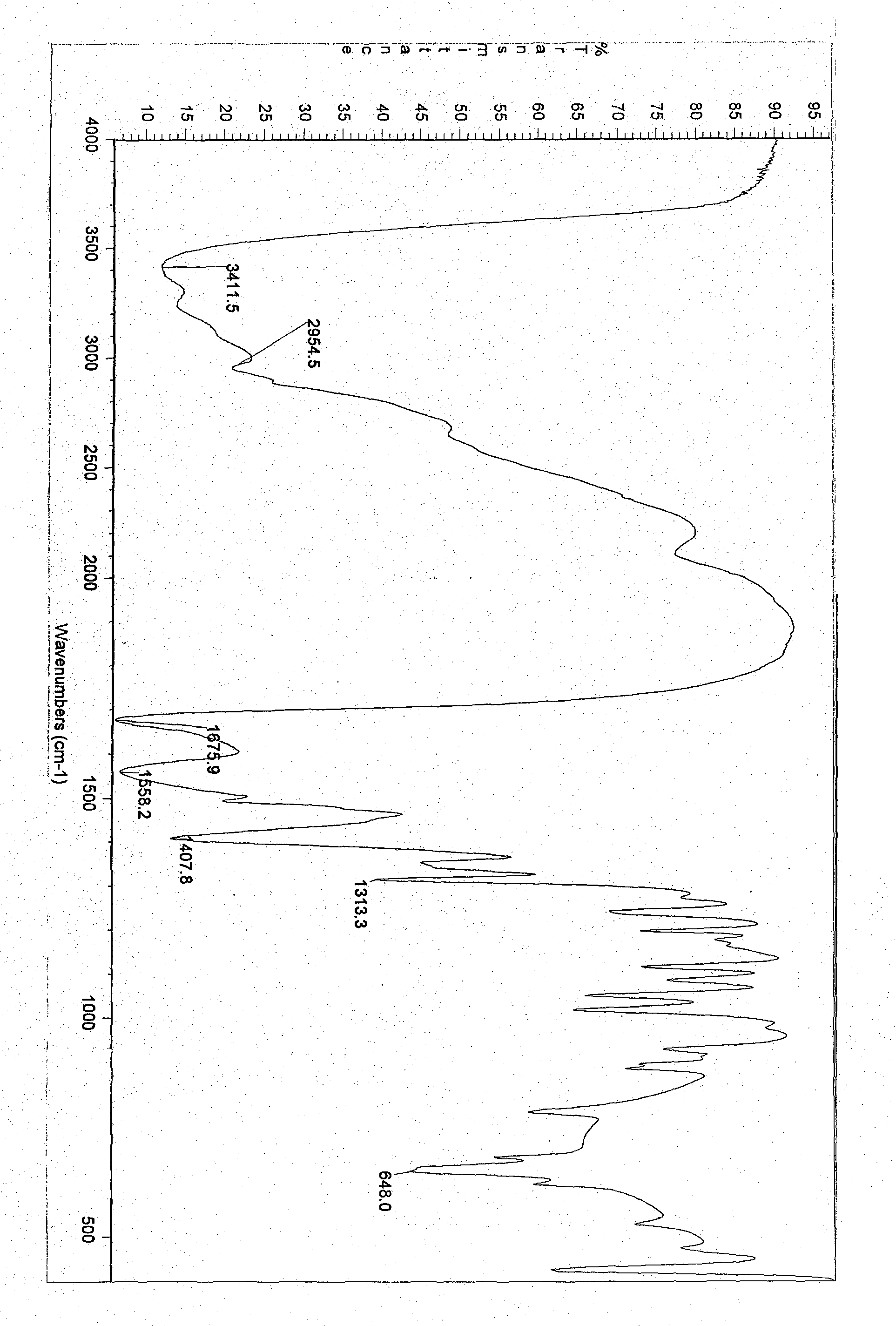
[0047] Molecular formula: C 7 h 14 N 2 o 3 , Melting point: 127.9~130.9℃. IR(v max cm -1 ): 3411.5, 1675.9, 1558.2, 1407.8. 1 ...
Embodiment 2
[0049] Prepare Ornithine Aspartate Injection according to the following prescription:
[0050]
[0051]
[0052] Take 500.6g of ornithine aspartate, add 600mL of water for injection, stir to dissolve, add an appropriate amount of 1% acetic acid solution to adjust the pH to 5.7, add 0.1% activated carbon for needles, heat in a water bath at 40°C for 45 minutes, Filter through a 0.45 μm filter membrane, then rinse the activated carbon filter cake with a small amount of water for injection several times, and combine the collected filtrates. Dilute the filtrate to the 1000mL mark, accurately measure 10mL and pour it into a 10mL ampoule, seal, test, check for leaks, and sterilize at 115°C for 30 minutes. Take it out, cool it down to room temperature naturally, and make ornithine-aspartate injection.
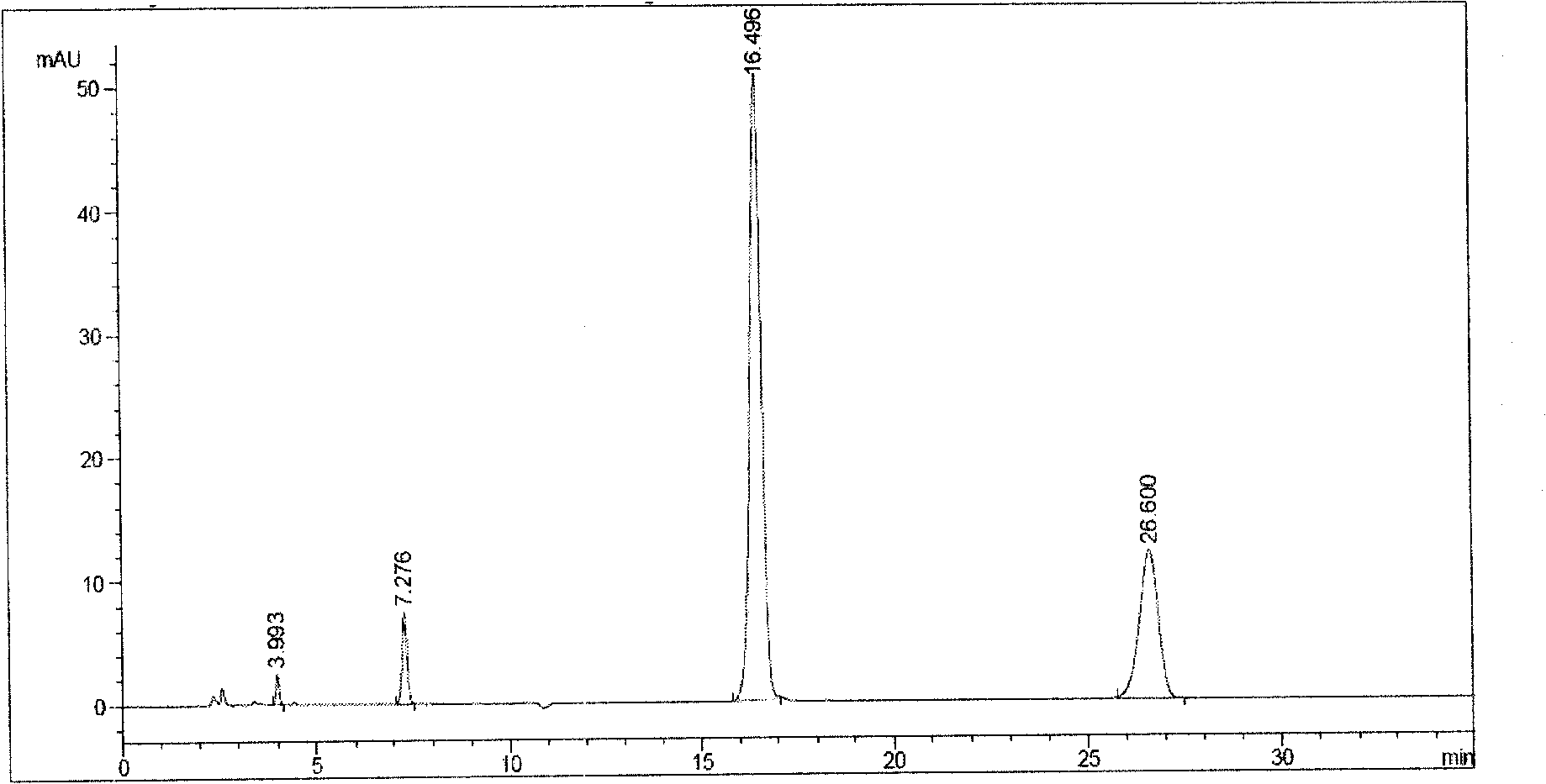
[0053] Product testing: pH=5.75; after HPLC analysis and calculation, the content of main impurities is 0.10%, and the content of related substances is 0.21%. Such as figure...
Embodiment 3
[0055] Prepare Ornithine Aspartate Injection according to the following prescription:
[0056]
[0057] Take 100.2g of ornithine aspartic acid, add 600mL of water for injection, stir to dissolve, add an appropriate amount of 1% acetic acid solution to adjust the pH to 5.4, add 0.05% activated carbon for needles, heat in a water bath at 60°C for 30 minutes, and keep warm Filter through a 0.45 μm filter membrane, then rinse the activated carbon filter cake with a small amount of water for injection several times, and combine the collected filtrates. Dilute the filtrate to the 1000mL mark, accurately measure 10mL and pour it into a 10mL ampoule, seal, test, check for leaks, and sterilize at 121°C for 15 minutes. Take it out, cool it down to room temperature naturally, and make ornithine-aspartate injection.
[0058] Product testing: pH=5.50; after HPLC analysis and calculation, the content of main impurities is 0.07%, and the content of related substances is 0.15%
[0059] S...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com