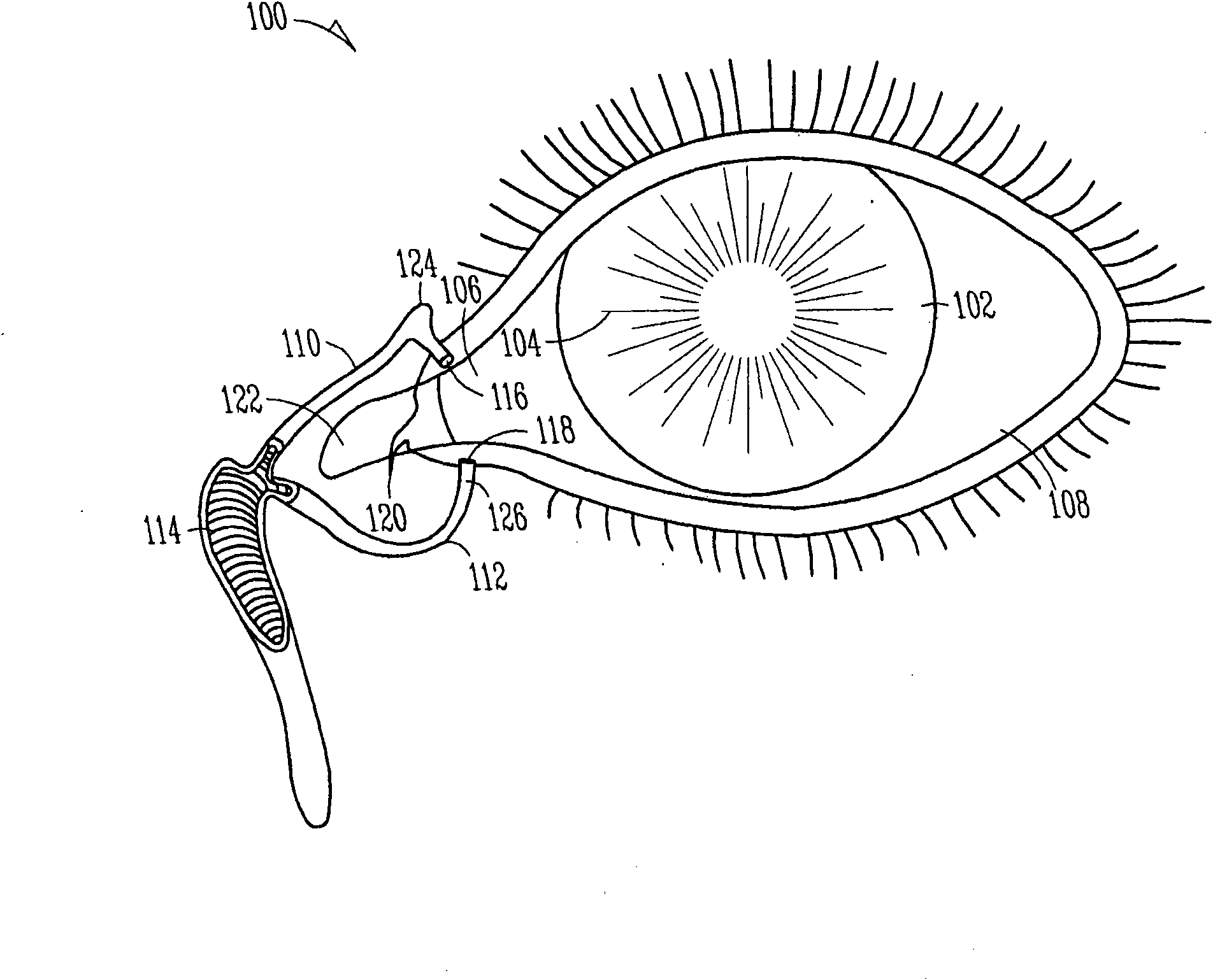
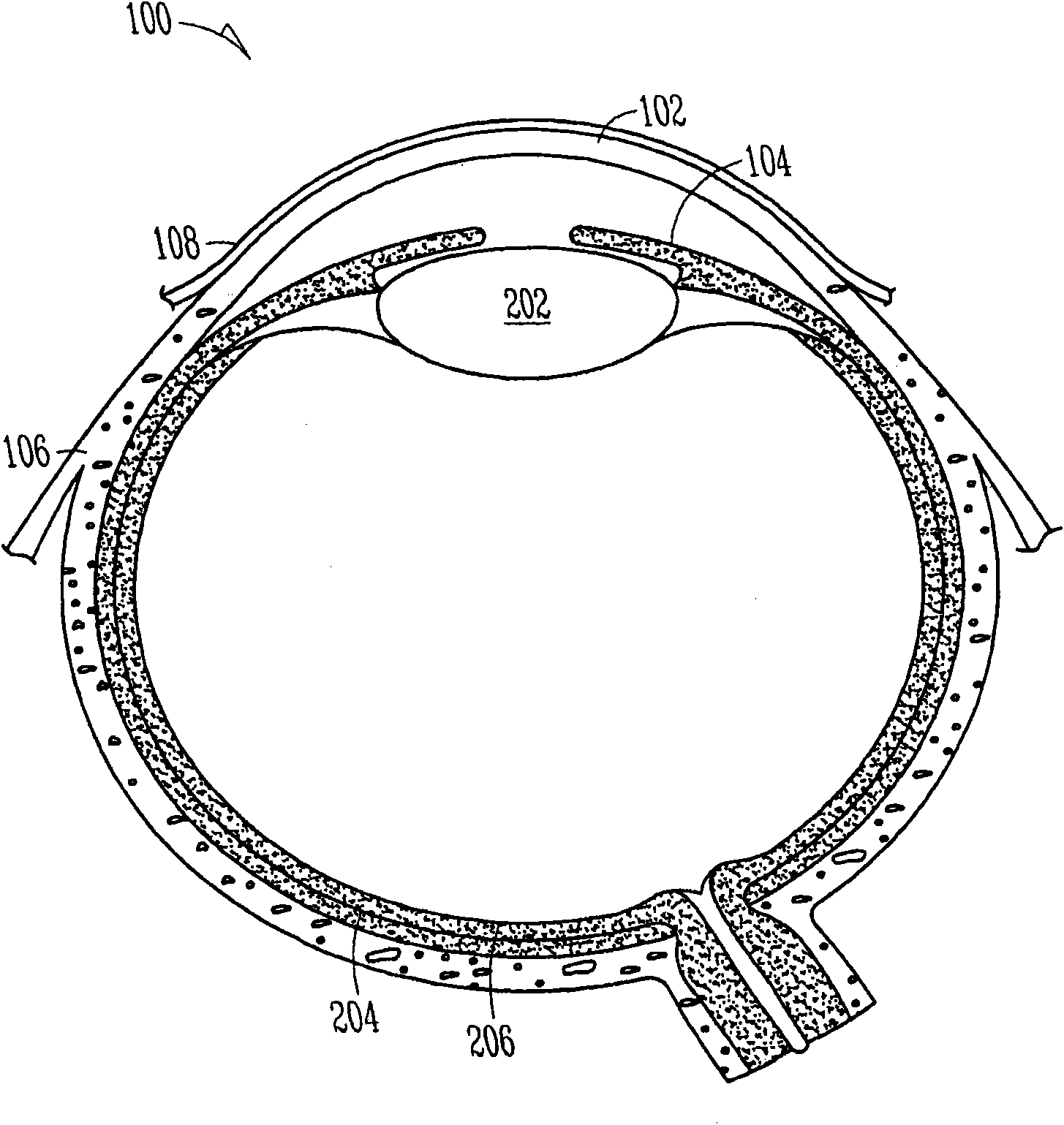
Lacrimal implants and related methods
A technology of lacrimal implants and implant bodies, which is applied in the field of eye implants and can solve the problems of liquid droplets missing the eyes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment Embodiment 1
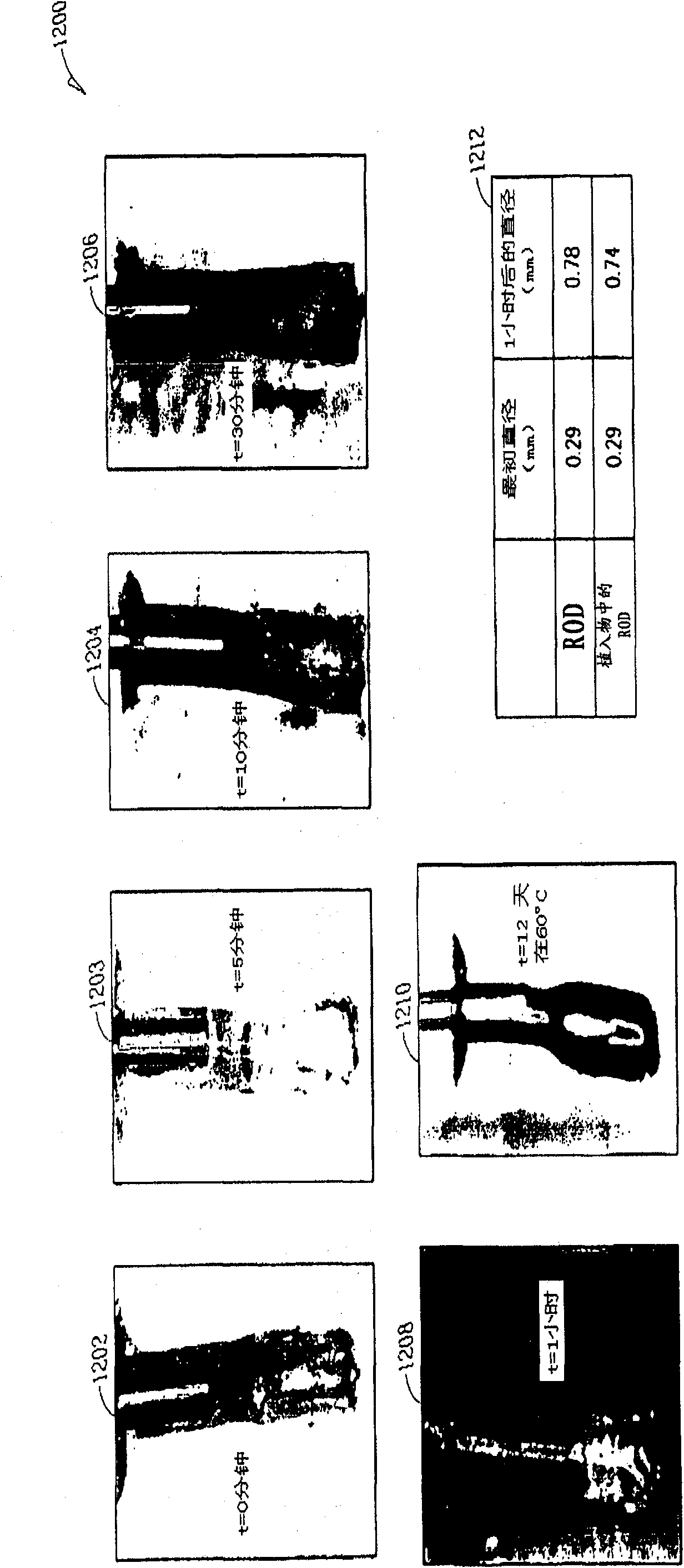
[0255] Figure 12 A lacrimal implant 1200 is illustrated that includes an implant body that includes a retention structure. In this example, the retention structure wraps around the Oasis hydrogel retention element. In order for the Oasis hydrogel retention element to receive fluid, the retention structure includes a fluid permeable retainer in the form of a silicone / contact lens hydrophilic cap component. In this example, the cap component comprised 80% silicone and 20% contact lens material (Vista Optics 75). In addition to allowing fluid to be received into the retention structure, the silicone / contact lens cap component additionally inhibits the Oasis hydrogel from sticking out of the retention structure during swelling.
[0256] At 1202, the Oasis hydrogel and package retention structure at t=0 minutes are shown. At 1203, the Oasis hydrogel and package retention structure at t=5 minutes are shown. At 1204, the Oasis hydrogel and package retention structure at t=10 min...
experiment Embodiment 2
[0258] Figure 13 A lacrimal implant 1300 is illustrated that includes an implant body that includes a retention structure. In this example, the retention structure encapsulates the TG-2000 hydrogel retention element. In order for the TG-2000 hydrogel retention element to receive fluid, the retention structure includes a fluid permeable retainer in the form of a silicone / contact lens hydrophilic cap component. In this example, the cap component comprised 80% silicone and 20% contact lens material (Vista Optics 75). In addition to allowing fluid to be received into the retention structure, the silicone / contact lens cap component additionally inhibits the TG-2000 hydrogel from sticking out of the retention structure during swelling.
[0259] At 1302, the TG-2000 hydrogel and encapsulated retention structures at t=0 minutes are shown. At 1304, the TG-2000 hydrogel and encapsulated retention structures at t=5 minutes are shown. At 1306, the TG-2000 hydrogel and encapsulated re...
experiment Embodiment 3
[0261] Figure 14 A lacrimal implant 1400 is illustrated that includes an implant body that includes a retention structure. In this example, the retention structure wraps the Contamac-78% hydrogel retention element. In order for the Contamac-78% hydrogel retention element to receive fluid, the retention structure includes a fluid permeable retainer in the form of a silicone / contact lens hydrophilic cap component. In this example, the cap component comprised 80% silicone and 20% contact lens material (Vista Optics 75). In addition to allowing fluid to be received into the retention structure, the silicone / contact lens cap component additionally inhibits the Contamac-78% hydrogel from sticking out of the retention structure during swelling.
[0262] At 1402, the Contamac-78% hydrogel at t=0 minutes and the encapsulated retention structure are shown. At 1404, the Contamac-78% hydrogel at t=5 minutes and the encapsulated retention structure are shown. At 1406, the Contamac-78%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com