Bombesin-oriented anti-tumor polypeptide and preparation method and application thereof
An anti-tumor, bombesin technology, applied in the application field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
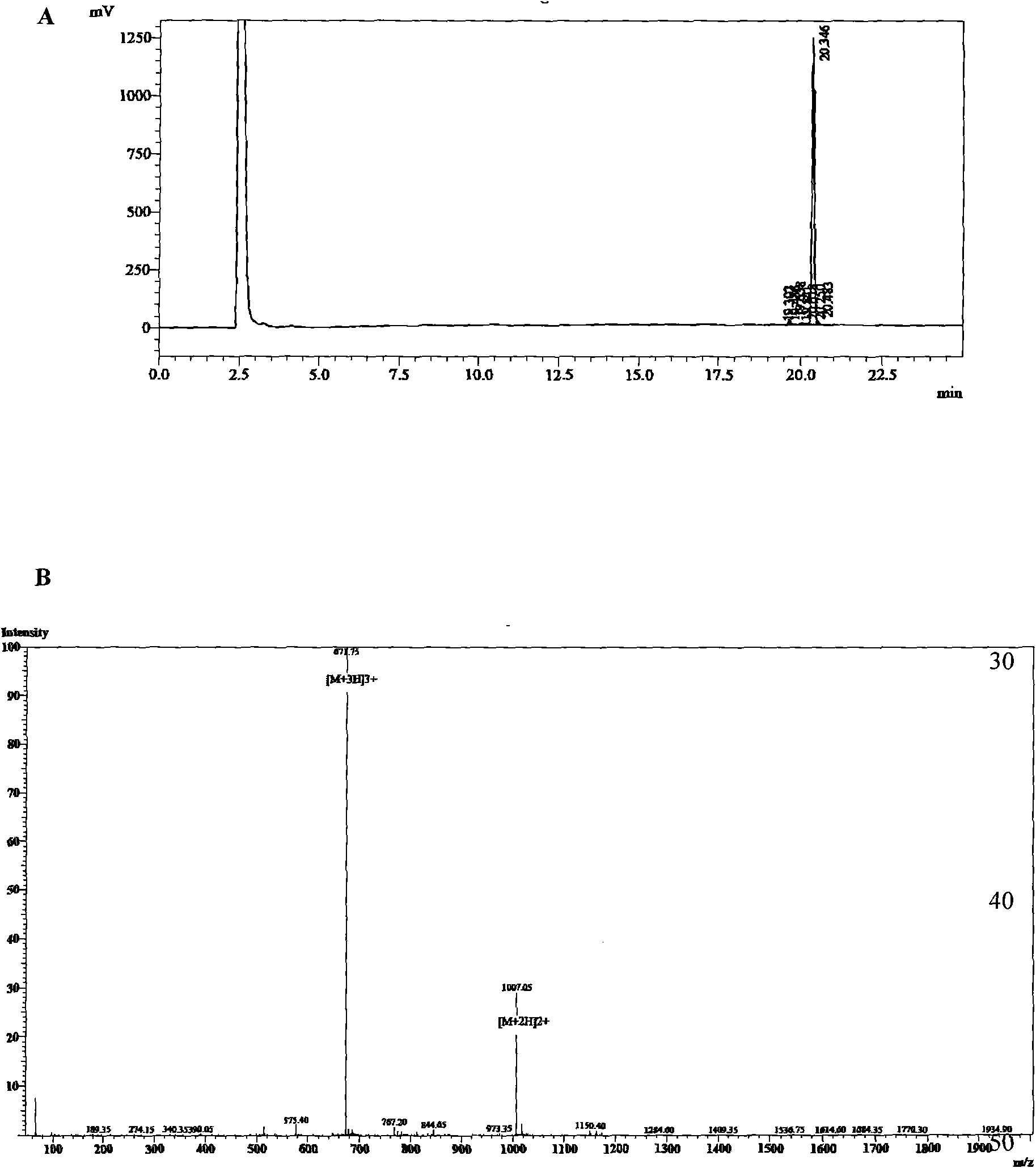
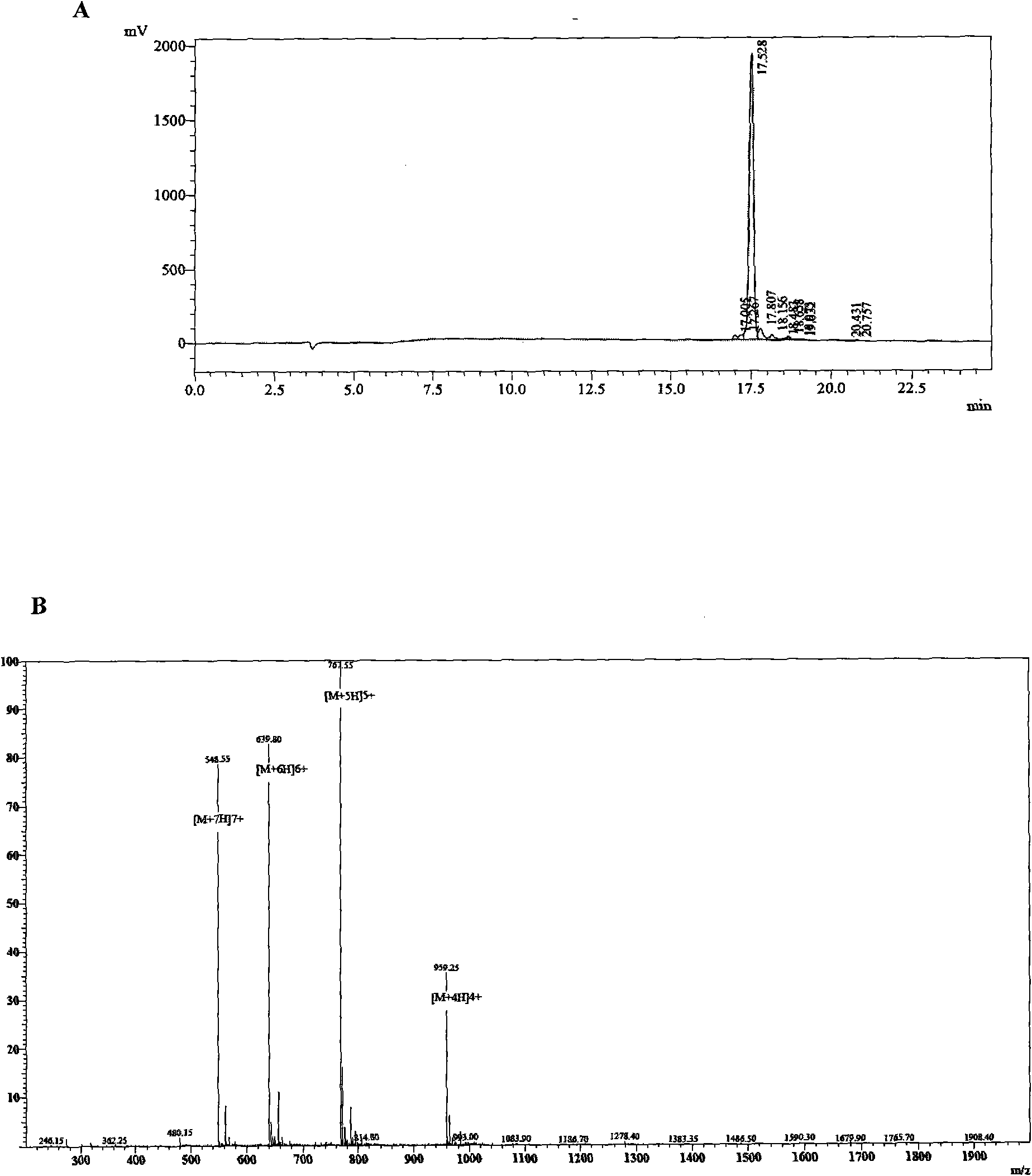
[0048] Embodiment 1 Preparation of antitumor peptide of the present invention
[0049] Unless otherwise specified, all cell lines of the present invention were purchased from the American ATCC cell bank. The cell lines used in the experiment include: human umbilical vein endothelial cells (HUVECs), vascular endothelial cells (ECV304), human embryonic lung fibroblasts (MRC-5), human prostate cancer cells (Du145), human breast cancer cells (MCF -7), human chronic myeloid progenitor cells (K562), human Burkitt's B lymphoma cells (Raji), human acute T lymphoma cells (CEM), human acute T lymphoblastic leukemia cells (Molt4), human acute leukemia early childhood granulocytes (NB4); and freshly isolated human decidual stromal cells (HDSCs). The peripheral blood lymphocytes of normal people were obtained from laboratory volunteers, and the peripheral blood lymphocytes of patients with acute myeloid leukemia were separated by gradient centrifugation using low-concentration Ficoll mono...
Embodiment 2 B28
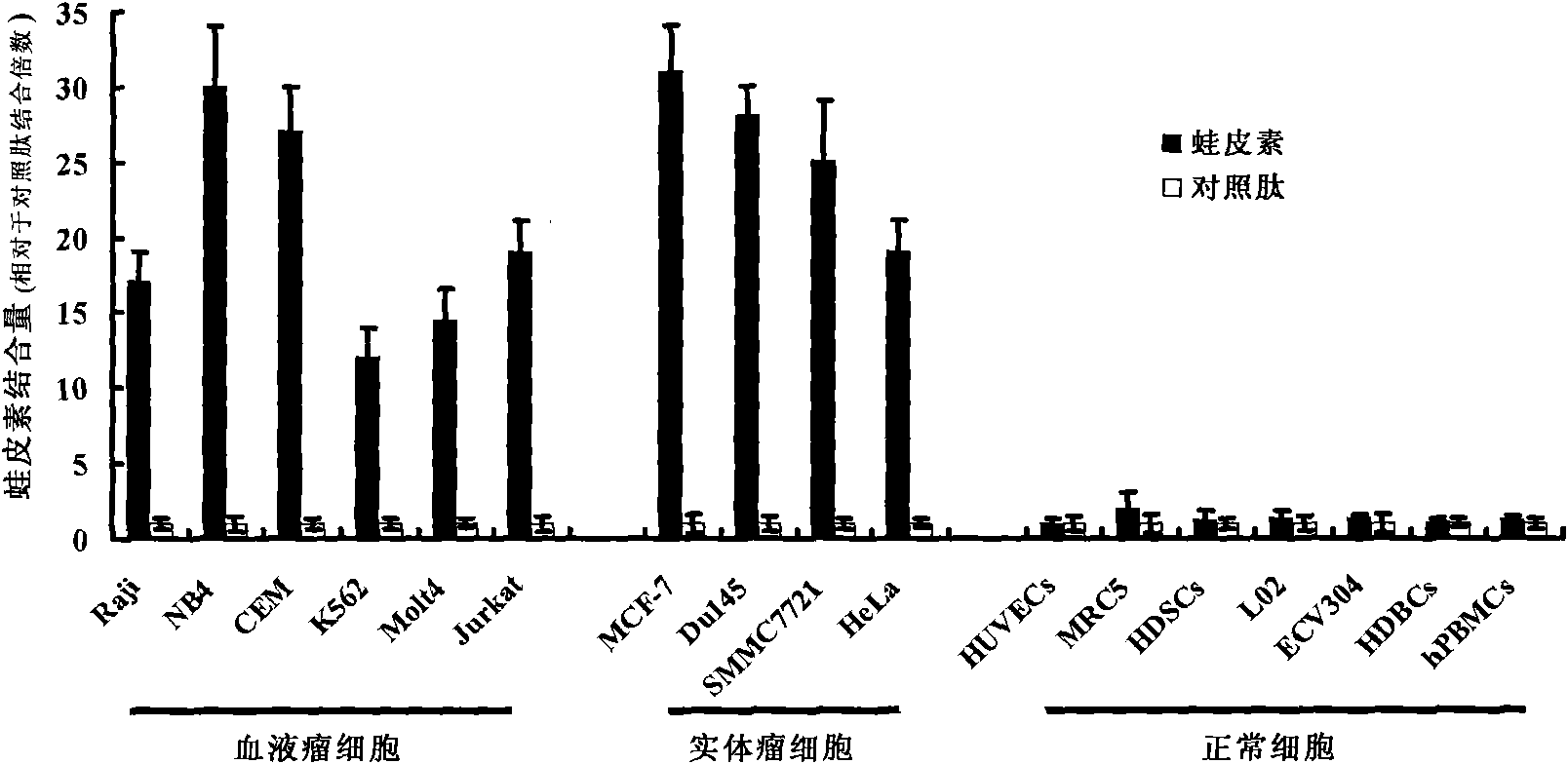
[0057] In vitro anti-tumor experiment of embodiment 2 BB28
[0058] The fusion peptide obtained in Example 1 was used to prepare peptide solutions of 2.5, 5, 7.5, 10, 12.5, and 15 μM, and the following experiments were carried out:
[0059] 1) Cytotoxicity test method
[0060] Adherent cells were seeded in 96-well plates at 10,000 cells / well and cultured overnight to allow them to adhere to the wall. On the next day, the supernatant medium was discarded and replaced with 100 μl medium containing 2% bovine serum albumin (BSA), and peptides of different concentrations were added for reaction. Non-adherent cells were suspended in 2% BSA medium, and after 10,000 cells / well were inserted into a 96-well plate, different concentrations of peptides were directly added. After the peptide acts on the cells, the CCK-8 kit can also be used to quantitatively determine the number of surviving cells by detecting the activity of mitochondrial enzymes in the cells. The specific operation is...
Embodiment 3 B28
[0079] Example 3 BB28 anti-tumor experiment in vivo
[0080] K562 cells (1×10 7 ) suspended in 100 μl of normal saline, and subcutaneously injected into 6-8 week old nude mice to establish a tumor model.
[0081] 1) Anti-tumor effect of intraperitoneal injection of BB28
[0082] When the tumor grows to 0.03-0.05mm 3 8 experimental animals were randomly divided into 2 groups. Animals in the experimental group were intraperitoneally injected with BB28 at a concentration of 20 mg / kg for 5 consecutive days. The control group was given the same volume of phosphate buffer. The diameter of the tumor was measured with a vernier caliper every day, and the volume of the tumor was calculated according to the formula (length x width x width x 0.5). After the experiment, the experimental animals were sacrificed, and the tumor tissues were taken out, photographed and weighed.
[0083] The result is as Figure 10 As shown in A, intraperitoneal injection of BB28 can significantly inhib...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com