Method for measuring relative absorption constants of dye, antibody, medicament and medicament prosoma molecules on polypeptide molecules
An adsorption constant, molecular technology, used in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
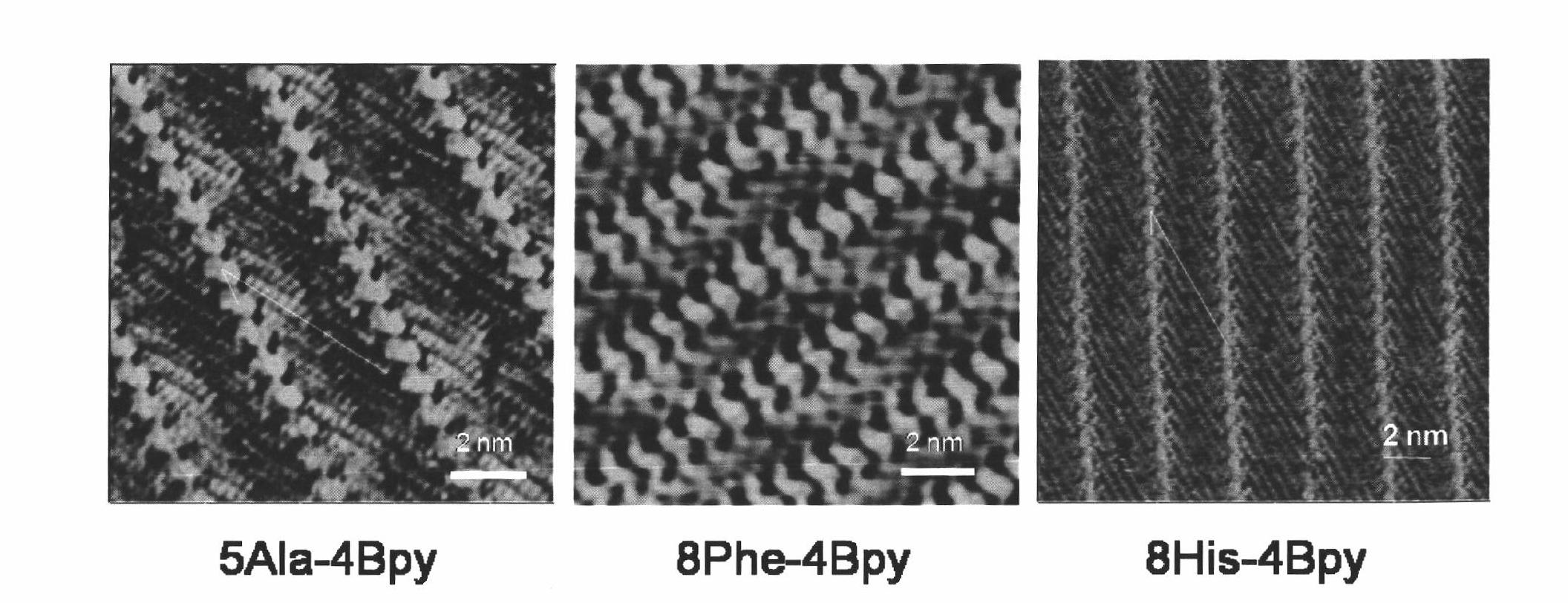
[0048] Example 1 Determining the Relative Adsorption Constant of Dye Molecules on Polypeptide Based on Scanning Tunneling Microscope
[0049] 1. The chemical structure of the substance used
[0050] The chemical structures of polypeptide molecules (5Ala, 8Phe and 8His), pyridine molecules (4Bpy) and dye molecules sulfophthalocyanine are as follows:
[0051]
[0052] Pentapolyalanine (5Ala)
[0053]
[0054] Octaphenylalanine (8Phe)
[0055]
[0056] Octahistidine (8His)
[0057]
[0058] 4'4-bipyridine (4'4-bipyridyl, 4Bpy)
[0059]
[0060] Phthalocyanine-tetrasulfonic
[0061] 2. Measurement method
[0062] First, mix the polypeptide molecules (5Ala, 8Phe and 8His) and the pyridine marker molecule 4Bpy in the aqueous solution, and ultrasonicate for 10 minutes. After mixing thoroughly, take out 15 microliters of the solution, drop it on the surface of the newly cleaved graphite, and let it stand for 10 minutes. After the mixed molecular system is assemb...
Embodiment 2
[0068] Example 2 Determination of Relative Adsorption Constant of Drug Molecules on Polypeptides Based on Scanning Tunneling Microscopy
[0069] 1. The chemical structure of the substance used
[0070] The chemical structures of the polypeptide molecule pentapolyalanine (5Ala), the pyridine marker molecule (4Bpy) and the drug molecule Congo red (Congo red) are as follows:
[0071]
[0072] Pentapolyalanine (5Ala)
[0073]
[0074] 4'4-bipyridine (4'4-bipyridyl, 4Bpy)
[0075]
[0076] Congo red
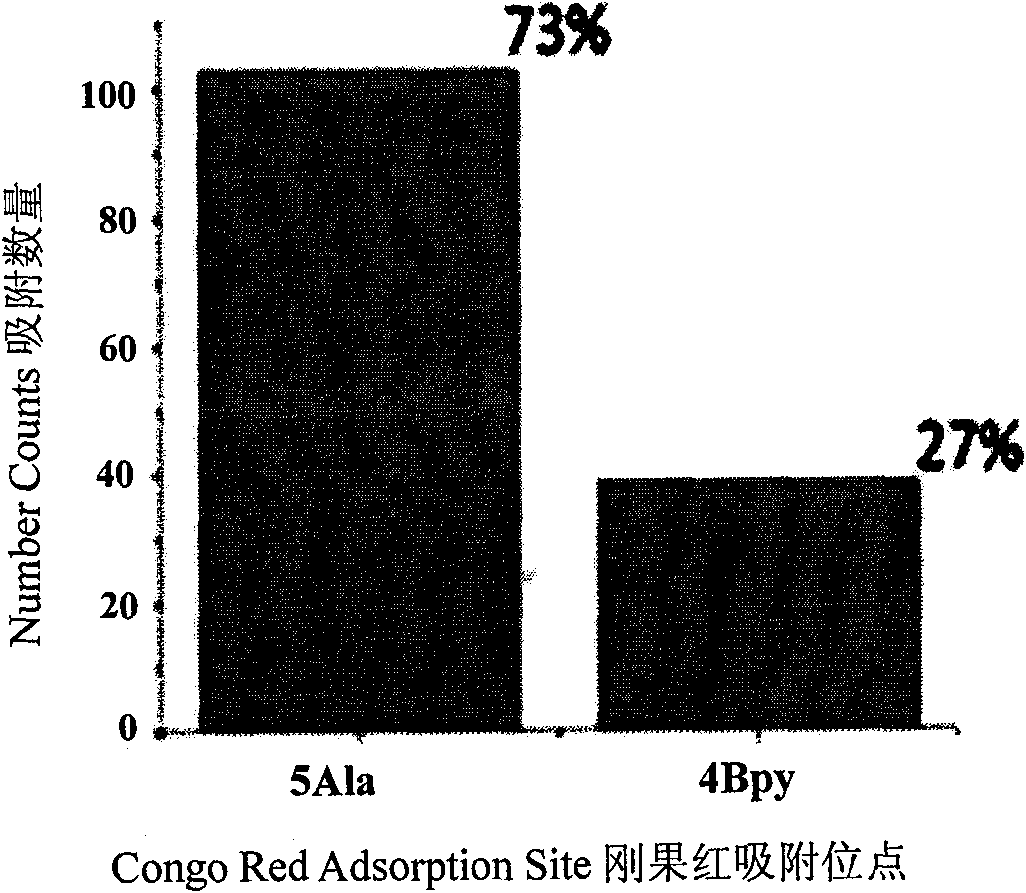
[0077] 2, assay method is identical with embodiment 1
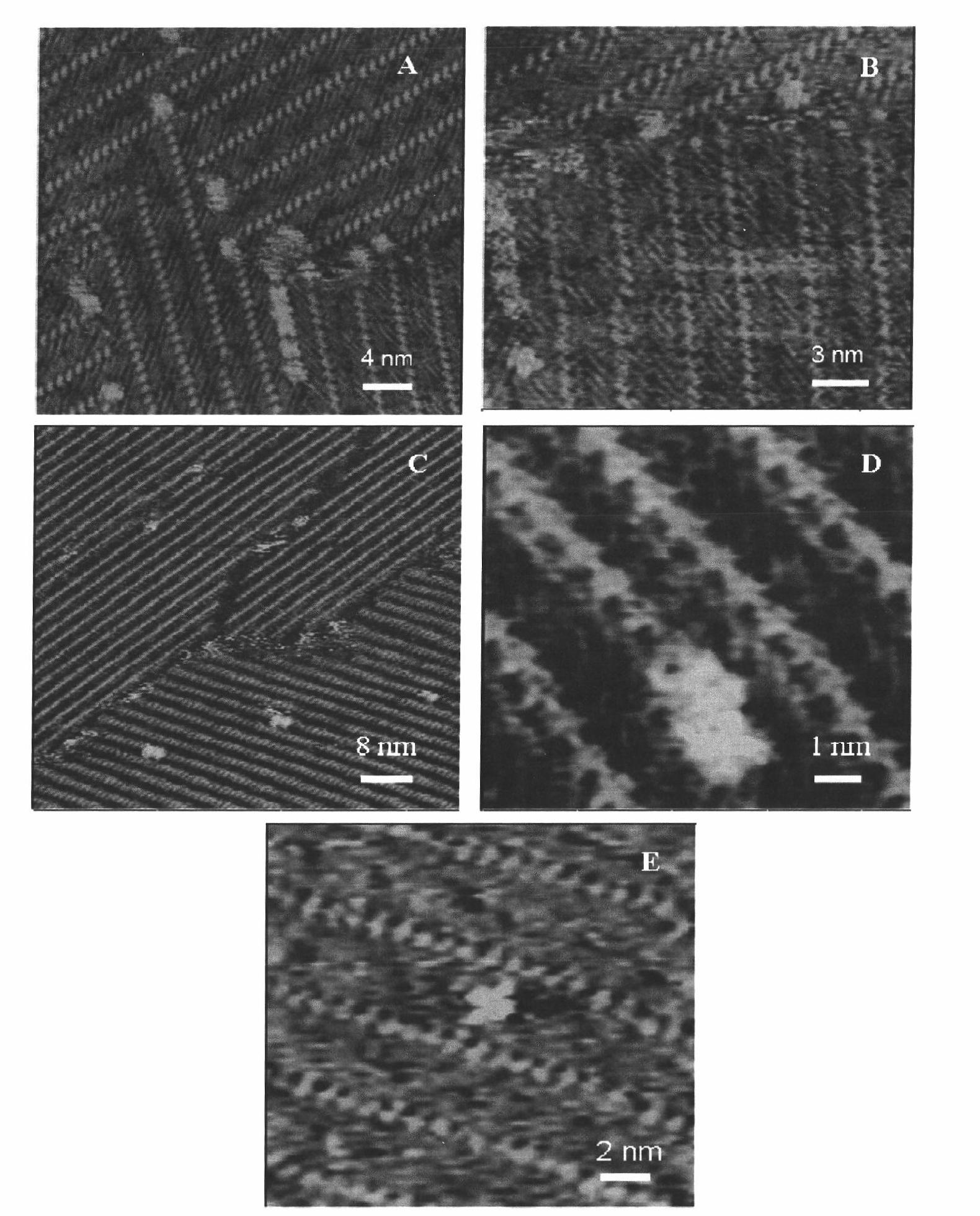
[0078] High-resolution STM images of Congo red adsorbed in the 5Ala and 4Bpy two-component assembly system were obtained by scanning tunneling microscopy (such as Figure 5 shown). By counting the amount of adsorption of Congo red on polypeptide and 4Bpy, the basis for the relative adsorption capacity of Congo red on polypeptide and marker molecule 4Bpy is provided (such as Figure 6 shown).
Embodiment 3
[0079] Example 3 Determination of Relative Adsorption Constant of Drug Prodrug Molecule on Polypeptide Based on Scanning Tunneling Microscopy
[0080] 1. The chemical structure of the substance used
[0081] The chemical structures of the polypeptide molecule pentapolyalanine (5Ala), the pyridine marker molecule (4Bpy) and the prodrug molecule Thioflavin (Thioflavin T, ThT) are as follows:
[0082]
[0083] Pentapolyalanine (5Ala)
[0084]
[0085] 4'4-bipyridine (4'4-bipyridyl, 4Bpy)
[0086]
[0087] Thioflavin (Thioflavin T, ThT)
[0088] 2. Measurement method
[0089] The assay method is the same as in Example 1, and a scanning tunneling microscope is used to obtain a high-resolution STM image (such as Figure 7 shown). By counting the adsorption quantity of ThT on the polypeptide and 4Bpy, the basis for the relative adsorption capacity of ThT on the polypeptide and the marker molecule 4Bpy is provided. Since ThT has no adsorption on 4Bpy, the relative ads...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com