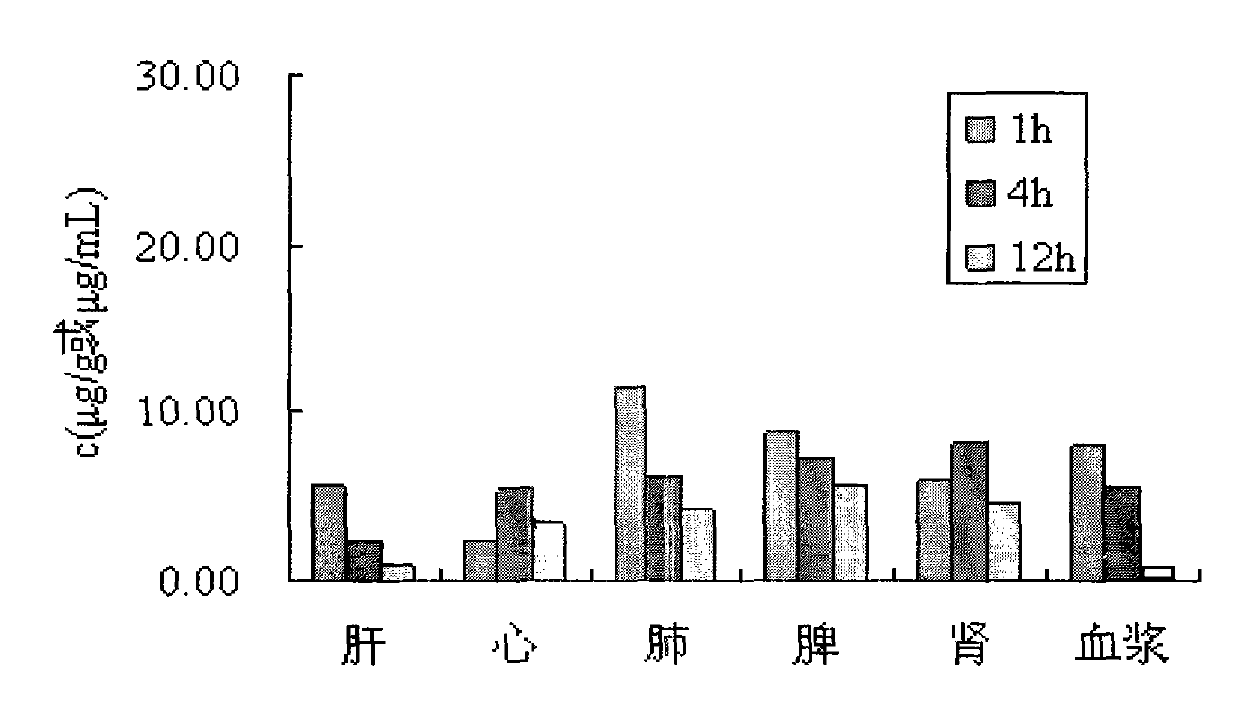
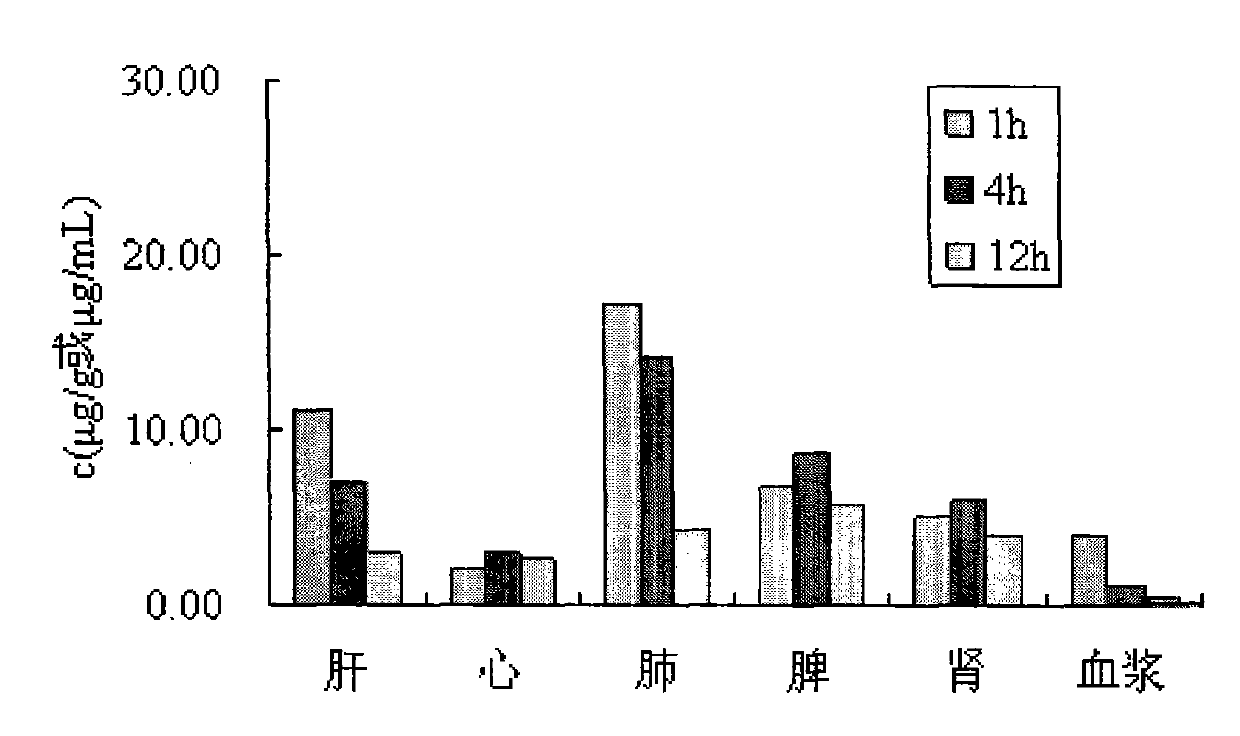
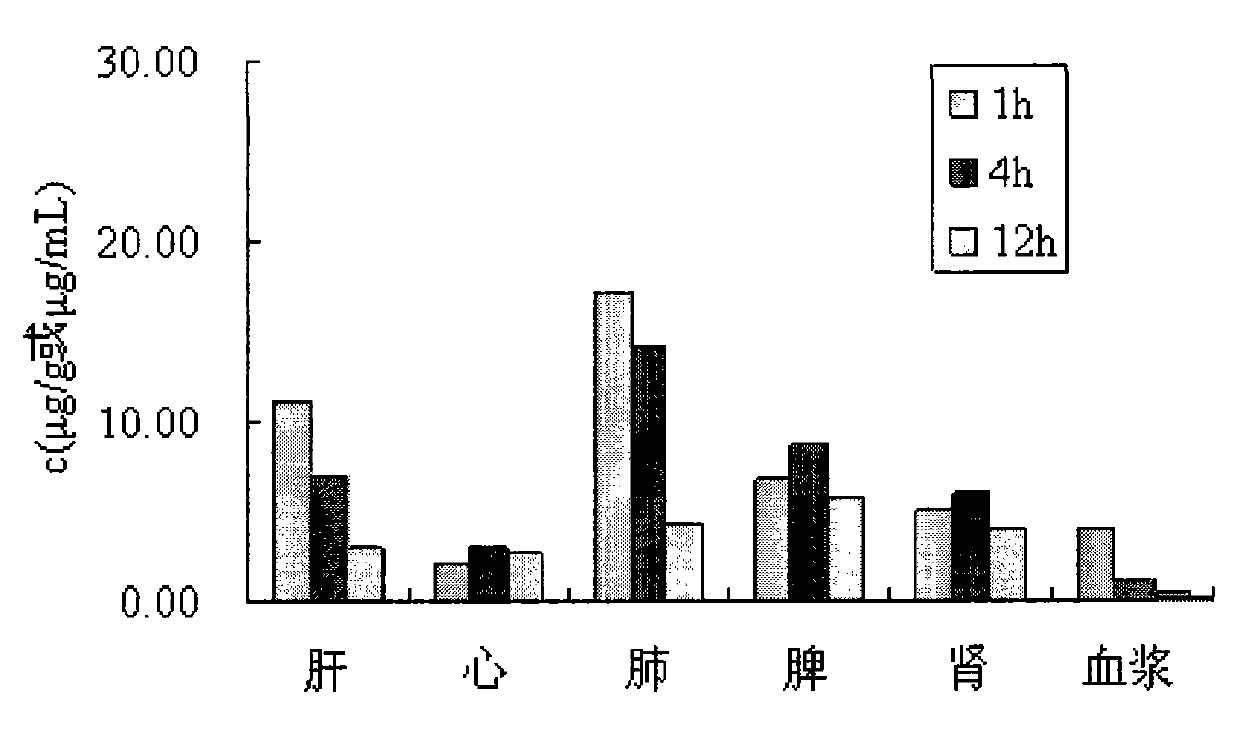
Taxol nanoparticle freeze-drying preparation containing recombinant human serum albumin
A human serum albumin and nanoparticle technology, which is applied in the field of nanoparticle preparations of albumin-binding antitumor drugs and its preparation, can solve the problems of large particle size of nanoparticles, reduced industrialization value, and inability to achieve intravenous drip. , to achieve the effect of improving stability and increasing the value of industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Add 0.3g of paclitaxel, 0.05g of vitamin E into 5-15ml of absolute ethanol (or 0.1-1ml of chloroform) to dissolve, 6.5g of soybean lecithin, 5g of recombinant human serum albumin, 10g of trehalose, add 100ml of water for injection to fully dissolve, and use phosphoric acid Sodium dihydrogen buffer salt to adjust the pH value to 5.5, fully dispersed with a high-speed disperser, emulsified by a high-pressure homogenizer to 50% with a particle size range of 100nm, sterilized and filtered, and quickly frozen to -30 degrees under sterile conditions. Freeze-dry until the water content is less than 5% to obtain the paclitaxel recombinant human albumin conjugate dry powder.
Embodiment 2
[0037] Add 1.2g of paclitaxel, 10g of soybean lecithin to 50ml of absolute ethanol (or 2-10ml of chloroform) to dissolve, add 20g of recombinant human serum albumin, 15g of soybean lecithin, 0.05g of sodium metabisulfite, 60g of trehalose, add 500ml of water for injection to fully dissolve, The buffer solution of sodium dihydrogen phosphate and disodium hydrogen phosphate is adjusted to a pH value of 5.8, fully dispersed with a high-speed disperser, homogenized and emulsified to 50% by a high-pressure homogenizer, and the particle size range is 80nm, sterilized and filtered, under sterile conditions Quickly freeze to -40°C and freeze-dry until the water content is less than 5% to obtain dry powder of paclitaxel recombinant human albumin conjugate.
Embodiment 3
[0039] Paclitaxel 0.5g, add 5-15ml absolute ethanol (or 0.5-2ml chloroform) to dissolve, soybean lecithin 15g, recombinant human serum albumin 20g, maltose 30g, add 150ml water for injection to fully dissolve, use citric acid buffer saline or hydrochloric acid Adjust the PH value to 6.0 with salt, fully disperse with a high-speed disperser, emulsify to 50% with a high-pressure homogenizer, the particle size range is 80nm, sterilize and filter, quickly freeze to -45 degrees under sterile conditions, and freeze-dry to moisture The dry powder of paclitaxel recombinant human albumin conjugate is obtained when the content is less than 5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap