Application of 1,2-diphenylethylene derivative in pharmacy
A technology of stilbene and derivatives, applied in 1 field, can solve problems such as loss of pain sensation, burning sensation of capsaicin, loss of response to harmful stimuli, etc., and achieve the effect of relieving pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
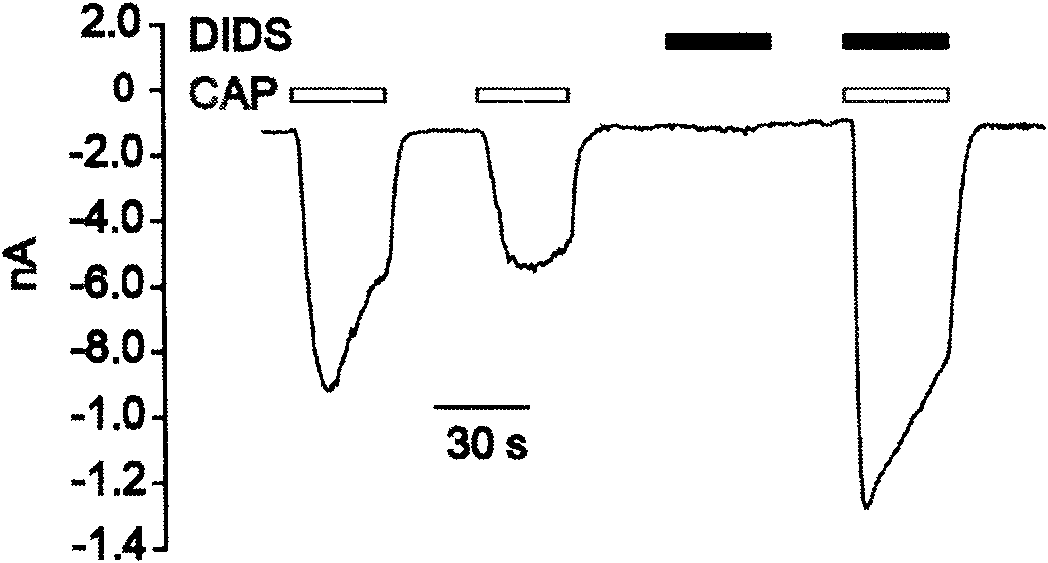
[0030] Example 1 The synergistic effect of DIDS on capsaicin (CAP for short) was determined by electrophysiological patch clamp.
[0031] (1) Test compound DIDS: commercially available DIDS CAS: 207233-90-7 (Sigma batch number: 068K1116)
[0032] (2) Test method: the culture of dorsal root neurons (DRG) of adult SD rats (200~250g): get the whole dorsal root ganglia of adult SD rats, digest with collagenase and trypsin, and prepare them in 10% fetal bovine serum , 100U / ml penicillin and streptomycin in DMEM culture solution blown, the cells spread on a 12mm round coverslip, cultured in a 24-well plate.
[0033] Patch clamp technique to record cell membrane current: Axon 700B was used as a patch clamp amplifier. Amphotericin B (final concentration: 0.1-0.2 mg / ml) was used in the electrode for punching patch-clamp recording. After the microelectrode is polished, fill the inner liquid of the electrode, and control the resistance value at 1.5-2.5MΩ. The electrode inner solution ...
Embodiment 2
[0035] Example 2 Electrophysiological Patch Clamp Assay (Sensitization of DIDS to Low pH Cell Solution Activated TRPV1 Channel)
[0036] (1) Test compound: DIDS
[0037] (2) test method: with the method used in embodiment 1
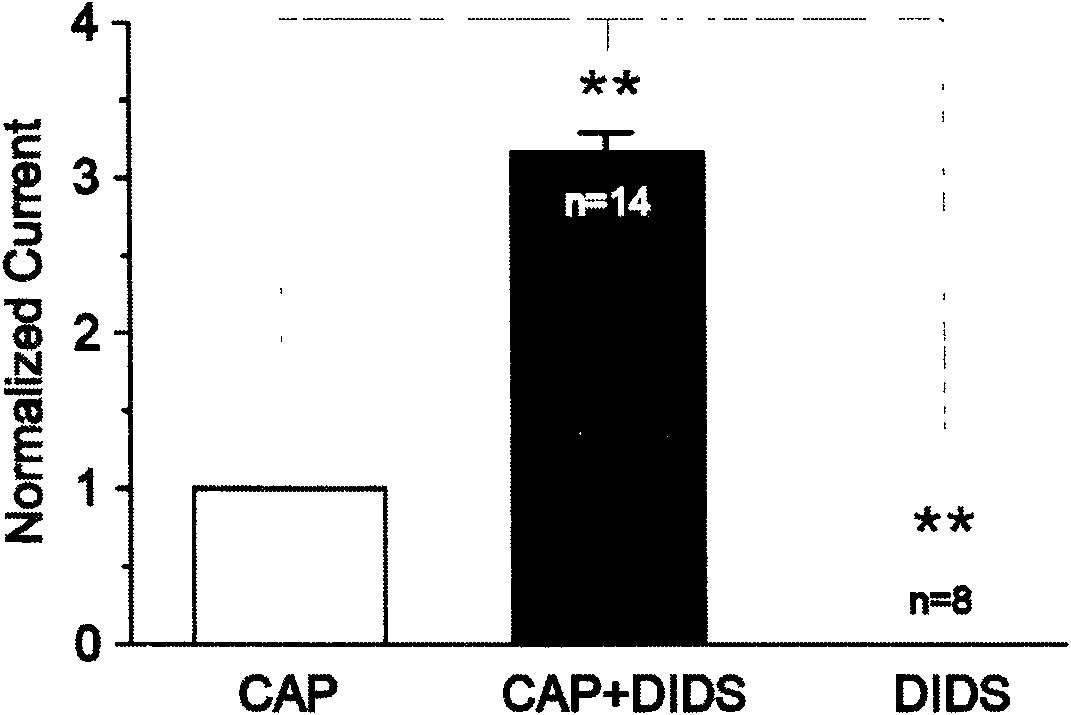
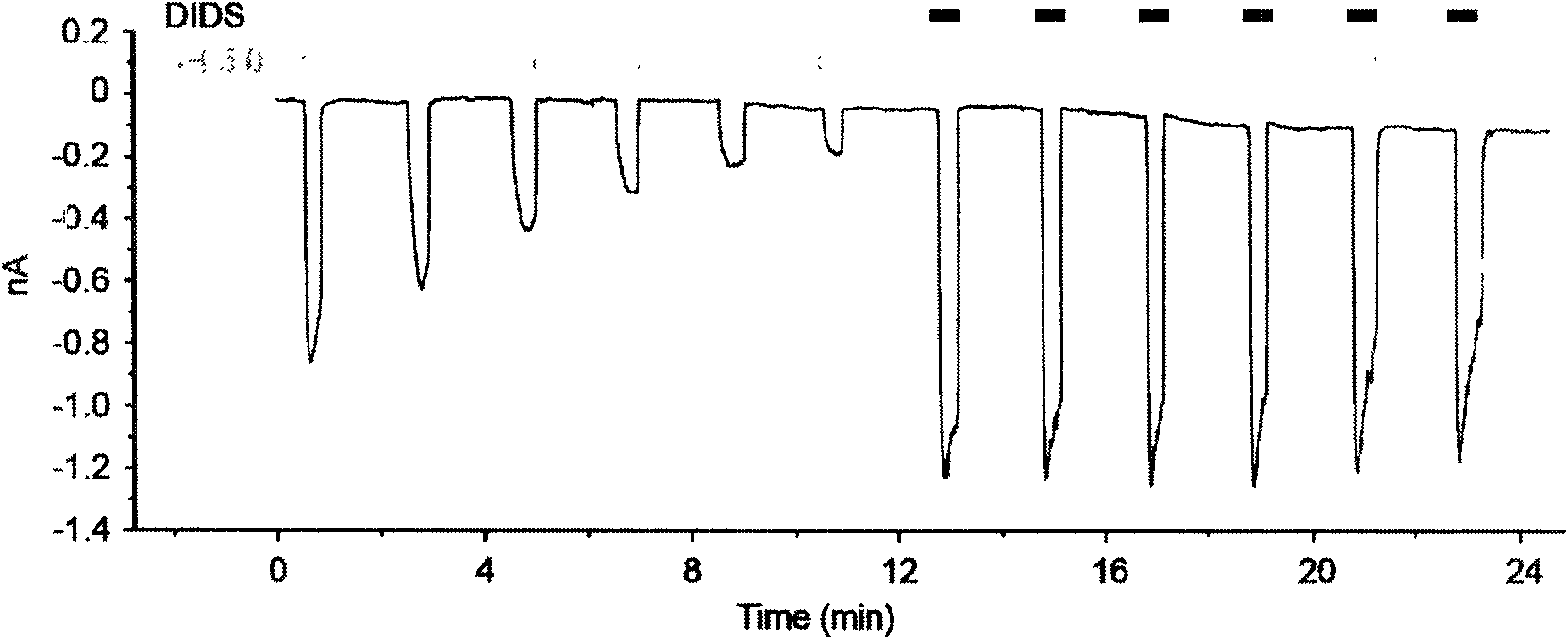
[0038] (3) Test results: if image 3 As shown, using the -60mV continuous recording mode to record the activation of the TRPV1 channel by repeatedly administering the extracellular fluid with pH=5, there is an obvious rapid desensitization phenomenon of the TRPV1 current. Combined application of 100 micromol DIDS on the basis of extracellular fluid at pH=5 can increase TRPV1 current and block the rapid desensitization of TRPV1 current. The combined application of DIDS and low pH cell fluid can cause the current to increase by 4.48±0.69 times (see Figure 4 ).
Embodiment 3
[0039] Example 3 Electrophysiological Patch Clamp Assay: Sensitization of SITS on Low pH Cell Solution Activation of TRPV1 Channel
[0040] The sources of the compounds described in the present invention can be obtained from commercial sources, and the specific source of the compounds described in the example of the determination of the biological activity of the compounds is DIDS CAS: 207233-90-7 (Sigma batch number: 068K1116);
[0041] (1) Test compound SITS, commercially available (SITS CAS: 51023-76-8 (Sigma batch number: 107K1065))
[0042] (2) test method: with the method used in embodiment 1
[0043] (3) Test results: The -60mV continuous recording mode was used to record the TRPV1 channel activated by the extracellular fluid at pH=5 for many times, and the TRPV1 current had obvious rapid desensitization phenomenon; the application of SITS alone could not trigger the TRPV1 current; Combined application of 100 micromolar SITS on the basis of external fluid can increase TR...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com