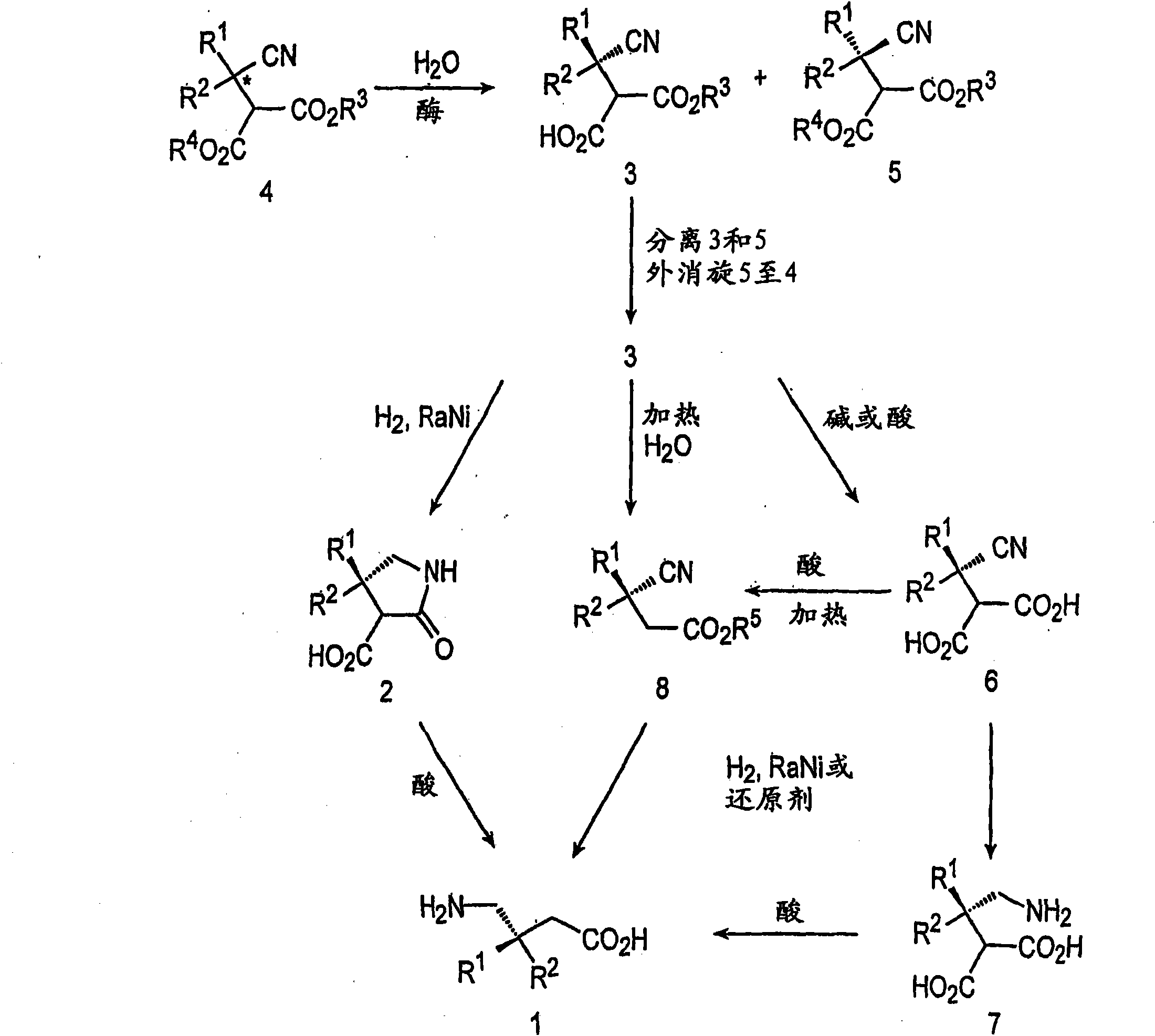
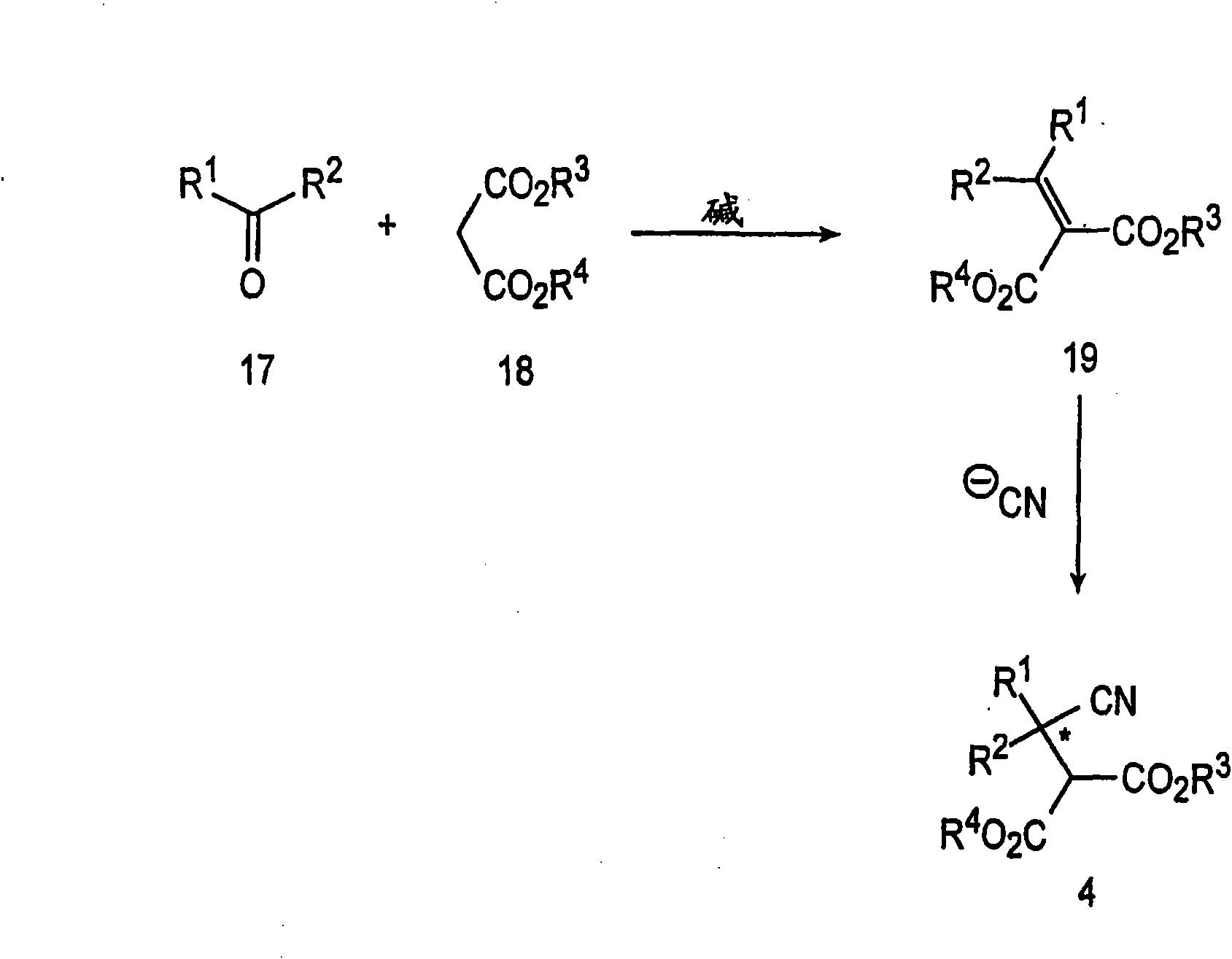
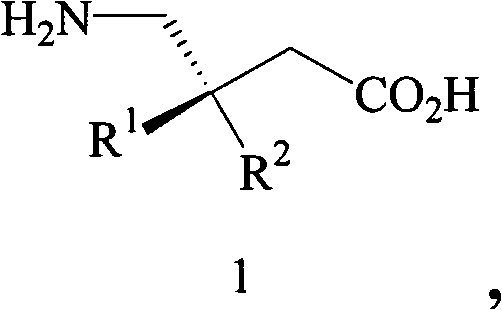
Preparation of pregabalin and related compounds
A technology of compounds and hydrates, applied in the field of γ-amino acids with binding affinity, can solve problems such as increased capital costs, difficulties in preparing bisphosphine ligands, and increased costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0210] Example 1. Generation of (3S)-3-cyano by enzymatic hydrolysis of (R / S)-3-cyano-2-ethoxycarbonyl-5-methyl-hexanoic acid ethyl ester (Formula 20) -2-Ethoxycarbonyl-5-methyl-hexanoic acid (Formula 21), for enzyme screening
[0211]
[0212] Enzyme screening was carried out using a screening kit comprising individual enzymes placed in separate wells of a 96-well plate previously prepared according to D. Yazbeck et al., Synth. Catal. 345: 524-32 (2003). Prepared by the method described. Each well has a void volume of 0.3ml (shallow well plate). One well of the 96-well plate contains only phosphate buffer (10 μL, 0.1M, pH 7.2), the other well contains only ACN (10 μL), and the remaining Each well contained one of the 94 enzymes listed in Table 2 (10 μL, 100 mg / mL). Before use, remove the screening kit from -80°C storage and allow the enzyme to thaw at room temperature for approximately 5 minutes. Using a multichannel pipette, potassium phosphate buffer (85 μL, 0.1 M, pH...
Embodiment 2
[0216] Example 2. Enzymatic resolution of (R / S)-3-cyano-2-ethoxycarbonyl-5-methyl-hexanoic acid ethyl ester (Formula 20) to generate (3S)-3-cyano- 2-Ethoxycarbonyl-5-methyl-hexanoic acid potassium salt (Formula 23) and (R)-3-cyano-2-ethoxycarbonyl-5-methyl-hexanoic acid ethyl ester (Formula 22)
[0217]
[0218] A reactor (392 L) equipped with overhead stirring was loaded with potassium phosphate buffer (292.2 L, 10 mM, pH 8.0) and 100L, EX type (3.9L). The mixture was stirred at 800 RPM for 1 min and KOH (2M) was added to adjust the pH to 8.0. Add (R / S)-3-cyano-2-ethoxycarbonyl-5-methyl-hexanoic acid ethyl ester (formula 20, 100kg), and during the hydrolysis, titrate the obtained with NaOH aqueous solution (50%) The mixture was maintained at pH 8.0. by HPLC (C 18 Column, 4.6mm x 150mm, detection at 200nm), monitors the extent of the reaction. After reaching about 40-45% conversion (eg, after about 24 h), the reaction mixture was transferred to a separatory funnel. T...
Embodiment 3
[0219] Example 3. Enzymatic resolution of (R / S)-3-cyano-2-ethoxycarbonyl-5-methyl-hexanoic acid ethyl ester (Formula 20) to generate (3S)-3-cyano- 2-Ethoxycarbonyl-5-methyl-hexanoic acid potassium salt (Formula 23) and (R)-3-cyano-2-ethoxycarbonyl-5-methyl-hexanoic acid ethyl ester (Formula 22)
[0220] A reactor (3.92 L) equipped with overhead stirring was loaded with calcium acetate buffer (1.47 L, 100 mM, pH 7.0) and (R / S)-3-cyano-2-ethoxycarbonyl-5-methyl- Ethyl hexanoate (Formula 20, 1 kg). The mixture was stirred at 1100 RPM for 5 minutes and KOH (5M) was added to adjust the pH to 7.0. join in 100 L, type EX (75 mL), during the hydrolysis, the resulting mixture was titrated with KOH (5M), maintaining pH 7.0. by HPLC (C 18 Column, 4.6mm x 150mm, detection at 200nm), monitors the extent of the reaction. After reaching about 42% to 45% conversion (eg, after about 20-25 h), the reaction mixture was transferred to a separatory funnel. The aqueous mixture was extracted ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com