Adaptive molecule for delivery of adenovirus vectors
An adenovirus and virus technology, applied in the field of peptides, can solve the problems of low cell specificity, unsuitable for in vivo methods, and time-consuming
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
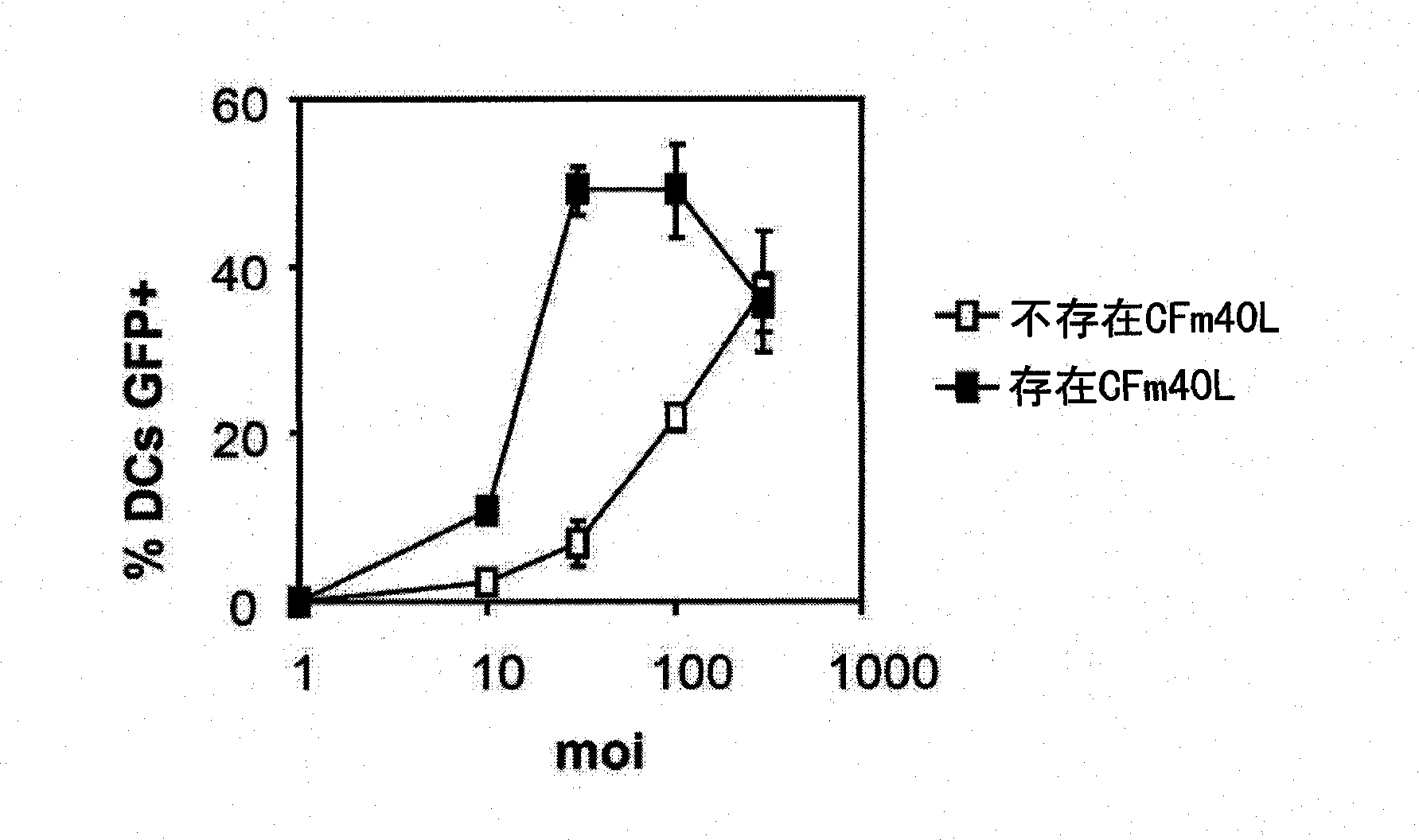
[0220] Example 1 Utilization of CFm40L to increase the efficiency of transduction of dendritic cells with adenovirus.
[0221] Dendritic cells (DCs) of C57BL6 mice were obtained from bone marrow precursors using the method described by Zabaleta et al. (Mol. Ther., 2008, 16:210-217). For this, femoral and tibial bone marrow was extracted using a lysis buffer solution (0.15M NH 4 Cl, 10mM KHCO 3 , 0.1 mM Na 2 EDTA) to lyse red blood cells. This was followed by washing with RPMI 1640, and lymphocytes and granulocytes were removed by incubating a mixture of antibodies against different cell populations and rabbit supplements.
[0222] - Anti-CD4 antibody (100 μg / mL): obtained from hybridoma GK1-5 (Dialynas et al., 1983, J Immunol. 1983 Nov; 131:2445-51).
[0223] - Anti-CD8 antibody (100 μg / mL): obtained from rat hybridoma H35.17.2 (Pierres et al., 1982, Eur. J. Immunol. 12 (1982), 60-69.).
[0224] -Ly-6G / Gr1 (BD Pharmingen; San Diego, Calif.), 10 μl / mL.
[0225] - CD45R / B2...
Embodiment 2
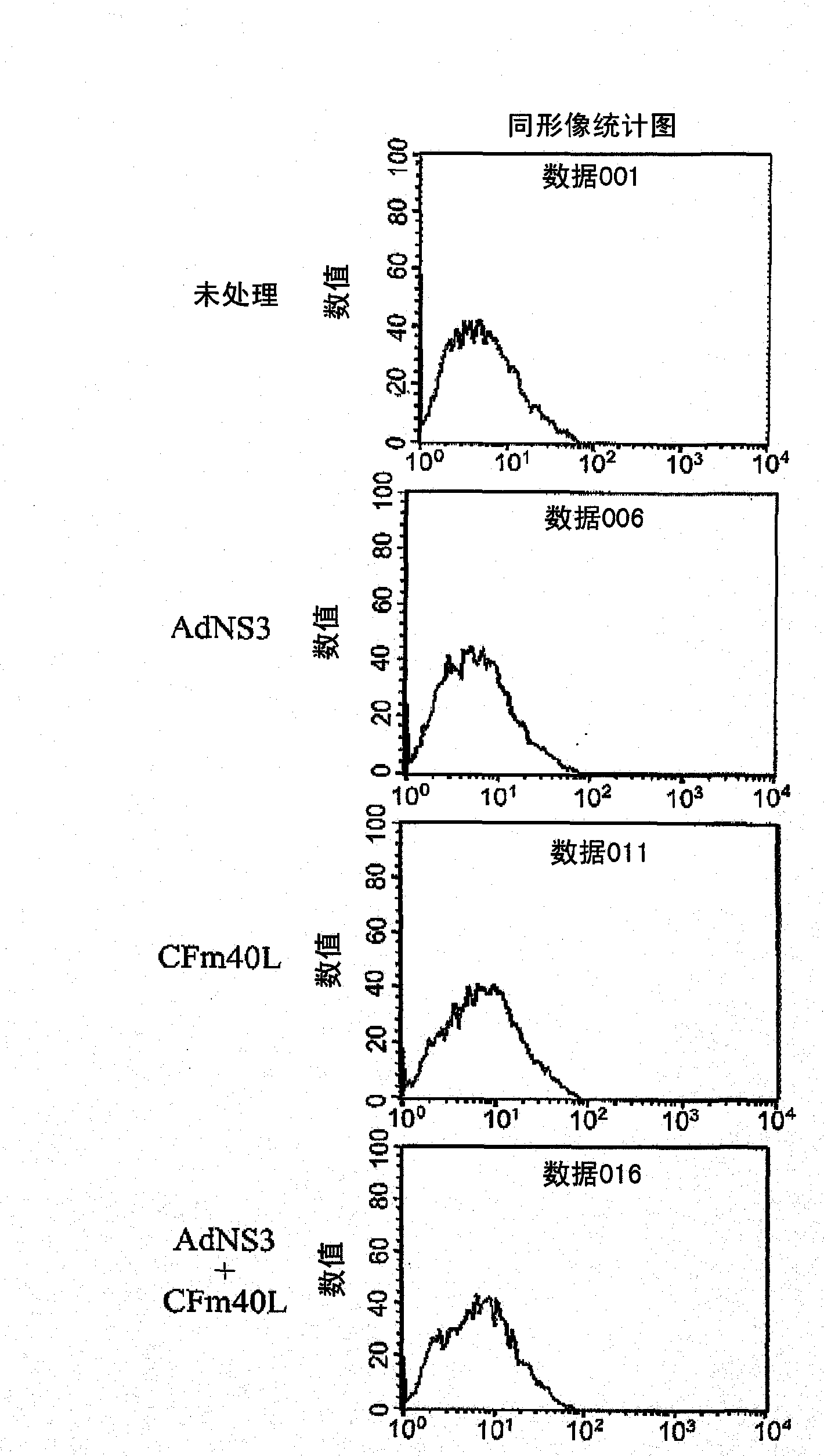
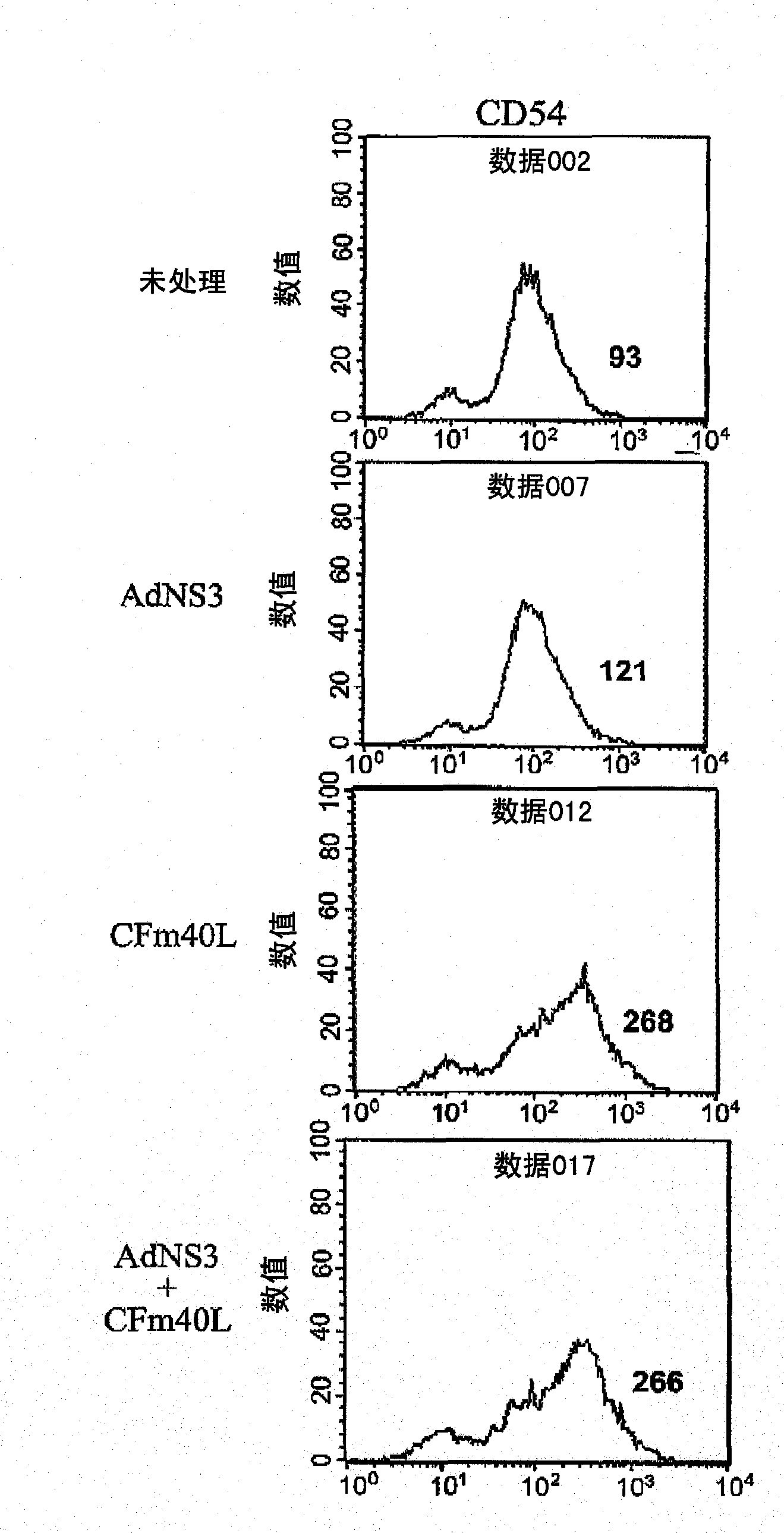
[0228] Example 2 Transduction of dendritic cells with AdNS3 in the presence of CFm40L to induce their in vitro maturation: expression of surface markers.
[0229]Dendritic cells from C57BL6 were prepared according to the method of Example 1 and transduced with AdNS3 (moi 30) in the presence or absence of CFm40L. Untreated dendritic cells or dendritic cells treated with CFm40L alone were used as controls. Dendritic cells were harvested one day later and their degree of maturation was studied by analyzing surface markers by flow cytometry. Antibodies against the markers CD54, CD80, CD86, I-Ab (MHC class II) were used, as well as controls (all from BD Pharmingen). Labeling was performed at 4°C in PBS with 2% FBS. After 30 minutes, cells were washed and analyzed for expression of different surface markers. The result is shown in Figure 2.
Embodiment 3
[0230] Example 3 - Transduction of dendritic cells with AdNS3 in the presence of CFm40L to induce their maturation in vitro: Cytokine production.
[0231] Dendritic cells were prepared according to Example 2, and divided into the same groups (untreated, AdNS3, CFm40L and CFm40L+AdNS3), cultured for 24 hours, and then the culture supernatant was collected. The amount of IL-12, IL-10 and IL-6 produced in these supernatants was determined by ELISA (BD-Pharmingen, Franklin Lakes, NJ, USA), performed according to the manufacturer's instructions. The result is as image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com