Preparation method for synthesizing graphene loaded noble metal catalyst in organic phase
A precious metal catalyst, a technology for synthesizing graphite, applied in catalyst activation/preparation, metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, etc. problems such as the speed of nanoparticle nucleation, to achieve the effect of convenient operation and prevention of overlapping
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0031] Example 1
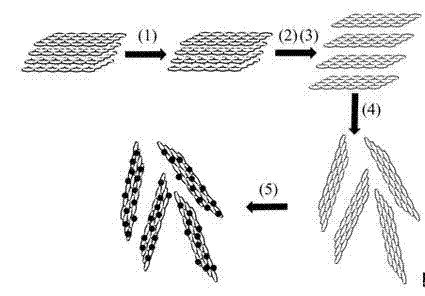
[0032] Such as figure 1 As shown, the preparation method described in this embodiment includes the following steps (precious metal precursor acetylacetonate palladium, stabilizer oleylamine and trioctyl phosphine are particularly preferred):
[0033] 1. In a round-bottom flask, add 50 mL of concentrated sulfuric acid (92 g), heat it to 80-90°C in an oil bath, and then add 10 g of potassium persulfate and 10 g of phosphorus pentoxide. 10g of natural graphite powder was slowly added to the above solution. This mixture was kept in an oil bath at 80-90°C for 4 hours. After cooling to room temperature, the mixture was diluted with deionized water, then vacuum filtered, washed with 3 L of deionized water, the solid was dried under vacuum for more than one day, and finally pre-oxidized graphite powder was obtained;
[0034] 2. In a 2L beaker, add 230 mL (4.3 mol) concentrated sulfuric acid, cool to 0°C, then add 5 g (0.42 mol) the first step pre-oxidized graphite powde...
Example Embodiment
[0038] Example 2
[0039] 1. In a round-bottom flask, add 50 mL of concentrated sulfuric acid (92 g), heat it to 80-90°C in an oil bath, and then add 16 g of potassium persulfate and 16 g of phosphorus pentoxide. 20g of natural graphite powder was slowly added to the above solution. This mixture was kept in an oil bath at 80-90°C for 4 hours. After cooling to room temperature, the mixture was diluted with deionized water, then vacuum filtered, washed with 3 L of deionized water, the solid was dried under vacuum for more than one day, and finally pre-oxidized graphite powder was obtained;
[0040] 2. In the beaker, add 421 mL (2.15 mol) concentrated sulfuric acid, cool to 0°C, then add 6 g (0.5 mol) the first step pre-oxidized graphite powder, and then 60 g (0.19 mol) potassium permanganate in batches Add it to a beaker while keeping the temperature below 10°C, and then keep it in a 38°C water bath for the best time for 2 hours. This mixture was diluted with 0.6L deionized water ...
Example Embodiment
[0044] Example 3
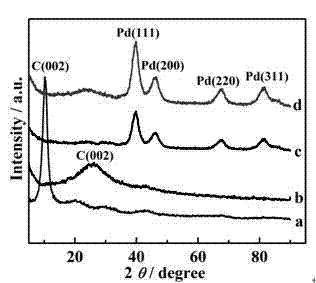
[0045] In a 50 mL round bottom flask, add 20 mL of N-methylpyrrolidone solution. Under nitrogen protection, add a certain amount of palladium acetylacetonate to the above solution. Under fully stirring, use a heating mantle to heat it After reaching the optimal reaction temperature of 180-200°C, after reacting for 2 hours and cooling to room temperature, add 20 mL of ethanol to obtain a black precipitate. After washing with a large amount of ethanol and acetone, dry to obtain palladium nanoparticles. figure 2 (C), the result shows that the simple substance of palladium was successfully obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Peak current | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com