Culture medium additive and application thereof
A medium additive, complete medium technology, applied in cell culture active agent, culture process, tissue culture and other directions, can solve the problems of slow reprogramming process, decreased reproducibility, serum instability, etc., to accelerate the iPS process , The effect of improving the efficiency of iPS
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0177] The process of cell experiment and virus preparation is as follows: the cells are planted in 12-well adherent cell culture plate, and the planting density is 20000 cells / well. 6-18 hours after the cells were planted, the cells were infected with the virus containing the mouse reprogramming factor depending on the cell density and state. Infection is carried out in two rounds, the second round of infection is carried out 24 hours after the first round of infection, and the virus liquid is replaced with various culture liquids to be tested 24 hours after the second round of infection. The day of medium change was recorded as day 0 (D0); at different time points after infection, count the number of GFP fluorescent clones in the original well or use flow cytometry to analyze the ratio of GFP fluorescent cells according to the needs of the experiment.
[0178] The preparation process of the virus is as follows: the reprogramming factor plasmid cloned on the PMX vector is tra...
Embodiment 1
[0181] Example 1: Comparison of reprogramming efficiency and component concentration optimization between B3.0 and other culture schemes.
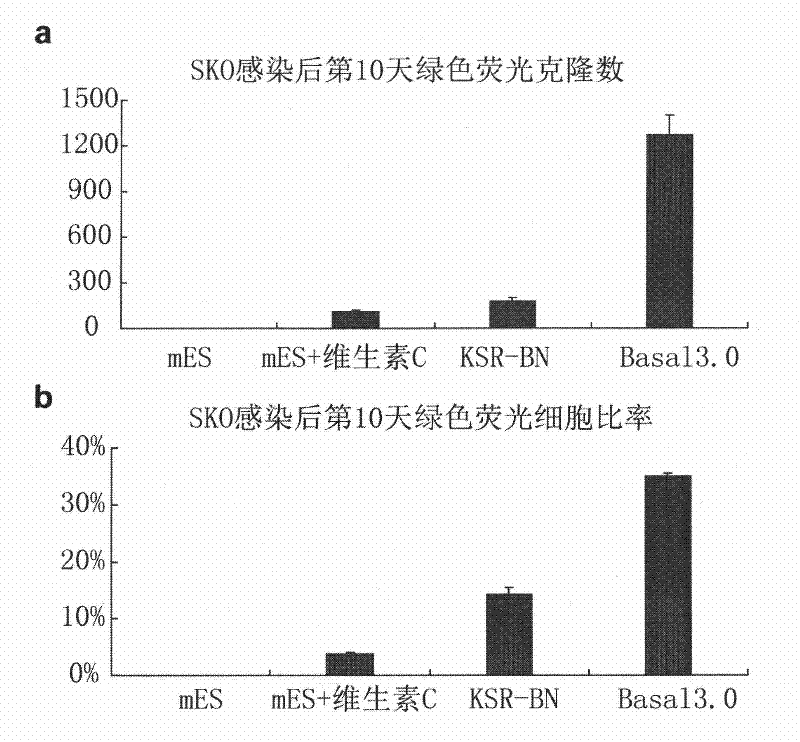
[0182] The SKO virus was mixed 1:1:1 (0.5ml each) and infected into a total of 20,000 fibroblasts in one well of a twelve-well plate, at 37 degrees, 5% CO 2 The conditions were cultured in mES medium, mES supplemented with vitamin C, KSR-BN medium and Basal3.0 medium. On the 10th day after infection, the number of reprogrammed clones was directly counted under a fluorescent microscope, and the ratio of reprogrammed cells was analyzed by flow cytometry. figure 1 a is the number of reprogramming clones induced by every 20,000 Oct4-GFP transgenic embryonic fibroblasts on the 10th day after infection under the culture conditions of mES and mES supplemented with vitamin C, KSR-BN and Basal3.04; The number of repetitions in each experimental group is n=3, and the error bars represent standard deviations. The results showed that on the 10th da...
Embodiment 2
[0186] Embodiment 2: Improvement to Basal3.0
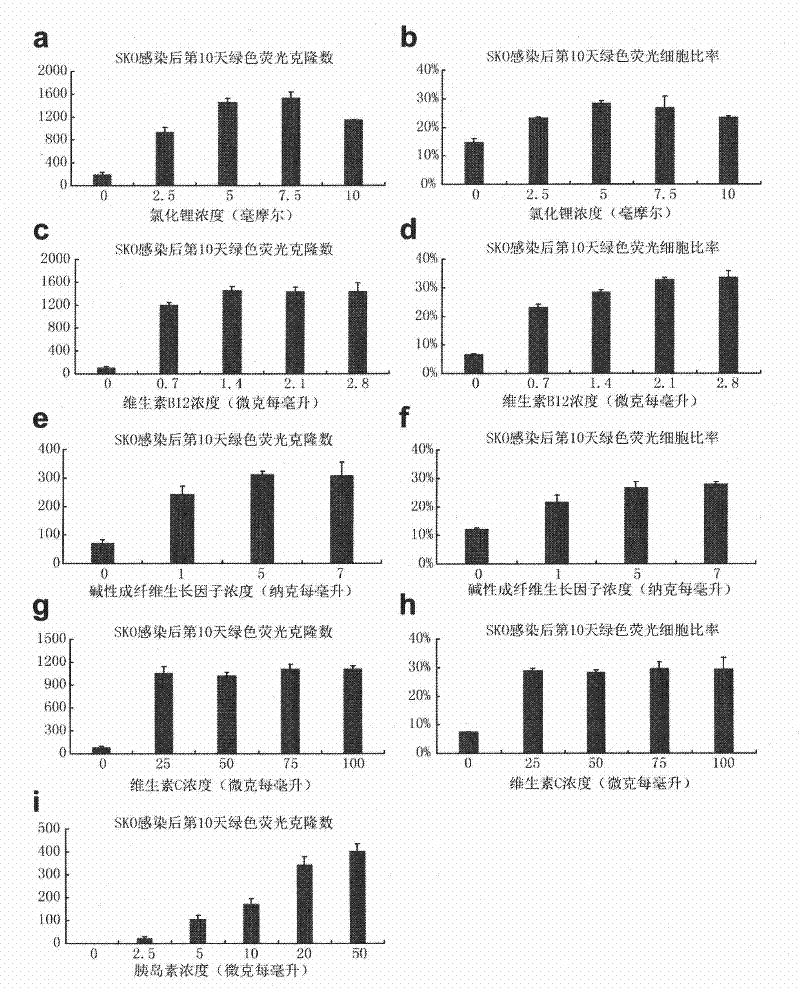
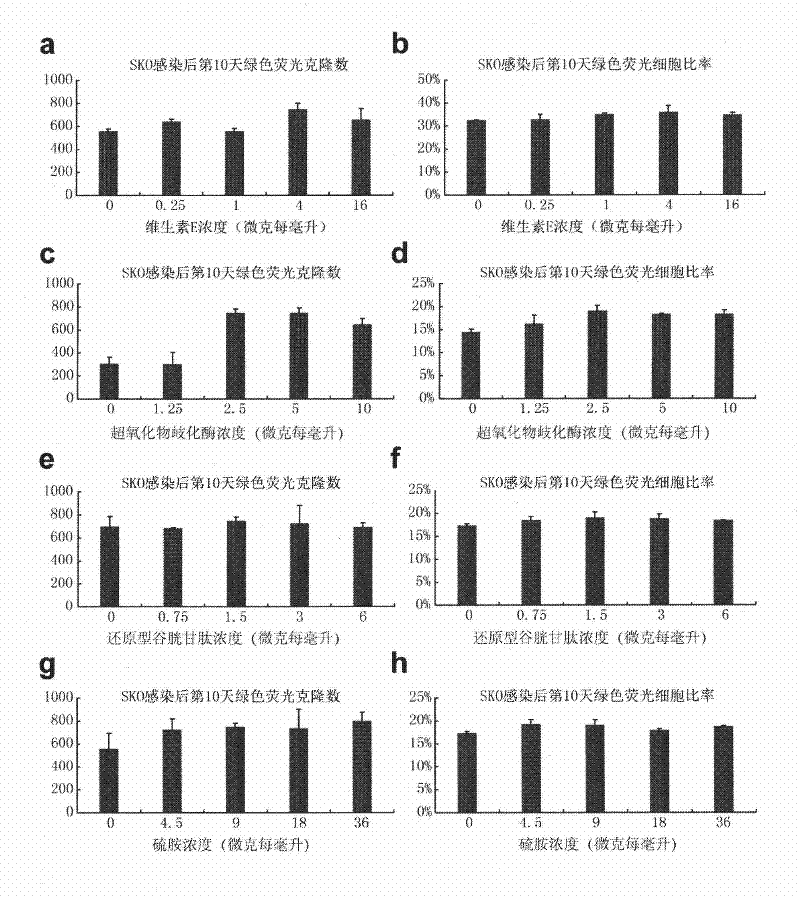
[0187] During the Basal3.0 concentration test, the significant effect of lithium chloride on reprogramming led us to speculate that the inhibition of glycogen synthesis kinase may be the reason for its promotion of reprogramming. Therefore, a series of glycogen synthesis kinase inhibitor substances include CHIR99021, BIO , SB31xxxx were used as candidate substances for the above iPS test experiments (the number of reprogrammed clones and the ratio of reprogrammed cells were used as evaluation criteria for reprogramming efficiency). As a result, the GSK3-β inhibitor CHIR99021 significantly improved the reprogramming efficiency of Basal3.0, so the improved Basal3.0 was added with the glycogen synthesis kinase 3 (GSK3-β) inhibitor CHIR99021, but not limited to adding the chemical composition of CH99021 The defined medium was named iCD1.
[0188] Through the above-mentioned iPS efficiency test experiment, we compared the reprogrammin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com