Dental compositions with fluorescent pigment
A technology of composition and organic pigments, applied in the direction of dentistry, dental prosthesis, dental preparations, etc., can solve problems such as reducing aesthetic quality, and achieve the effect of high resistance to fluorescence loss and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0096] Composition preparation and use
[0097] The dental compositions of the present invention can be prepared by mixing the various components using conventional mixing techniques. The resulting composition may optionally contain fillers, solvents, water, and other additives described herein. In general, the photopolymerizable compositions of the present invention are prepared by simply mixing the components of the compositions of the present invention under "safe light" conditions. Suitable inert solvents can be used, if desired, when affecting this mixture. Any solvent that does not react significantly with the components of the compositions of the present invention may be used. Examples of suitable solvents include acetone, dichloromethane, acetonitrile and lactones. A liquid material to be polymerized can be used as a solvent for another liquid or solid material to be polymerized. Solvent-free compositions can be prepared by simply dissolving the iodonium complex ...
example
[0105] As used herein,
[0106] "CAMP" refers to 1-(2-methacryloyloxyethyl)-2-(7-methylene-1,5,dithioct-3-yl)phthalate, such as WO / Prepared as described in Example 1 of US2006 / 122081;
[0107] "TrisMAP" refers to 2-[4-(2-hydroxy-3-methacryloxypropoxy)phenyl-2-[4-(2-[2-methacryloxyethoxy] Phthaloyloxy-3-methacryloxypropoxy)phenyl]-propane. It was prepared essentially as described in US Provisional Application No. 60 / 984,785, filed November 2, 2007 (Kalgutkar et al.).
[0108] "UDMA" means diurethane dimethacrylate available from Rohm America LLC, Piscataway, NJ under the trade designation "ROHAMERE 6661-0";
[0109] "BisEMA6" refers to ethoxylated bisphenol A dimethacrylate available from Sartomer Co., Inc., Exton, PA;
[0110] "TEGDMA" means triethylene glycol dimethacrylate, available from Sartomer Co., Inc., Exton, PA;
[0111] "BisGMA" means 2,2-bis[4-(2-hydroxy-3-methacryloxypropoxy)phenyl]propane;
[0112] "BHT" means butylated hydroxytoluene;
[0113] "SiO2 / ZrO2 Fi...
example E1 and E2
[0147] Examples E1 and E2 and Comparative Examples CE-1 to CE-5
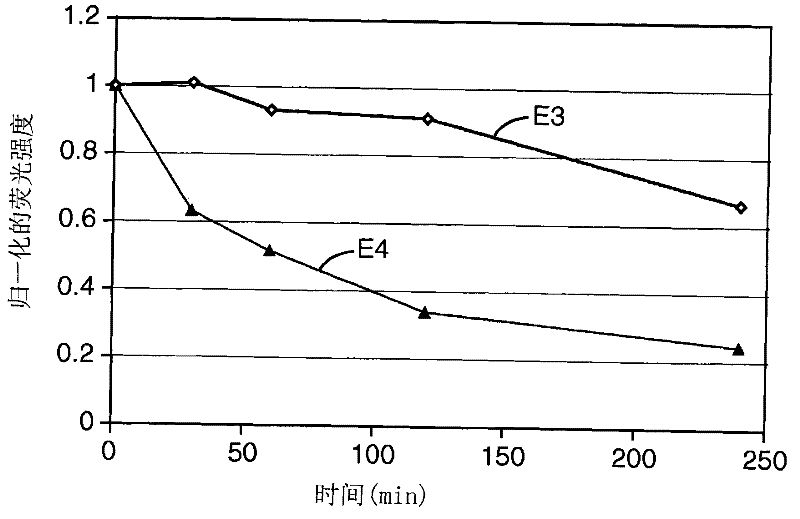
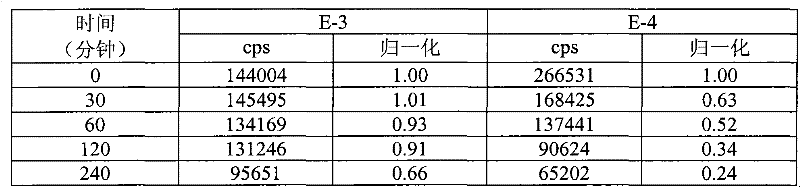
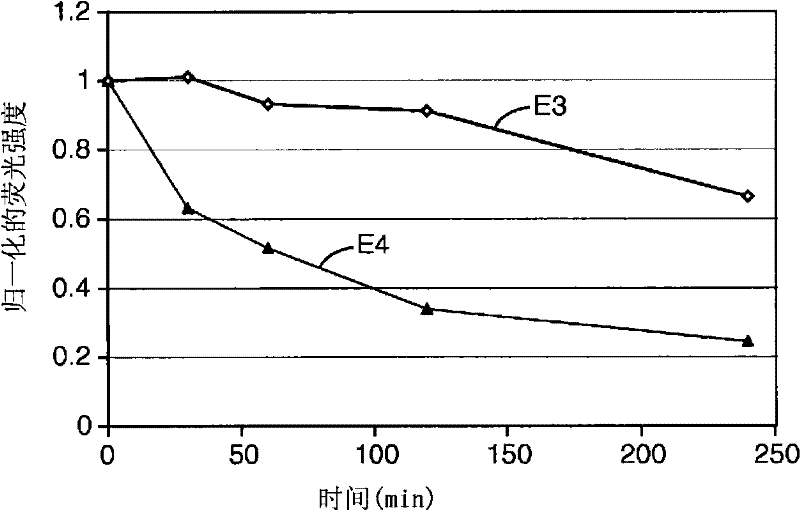
[0148] Composite materials shown in Table 1 were prepared by general techniques as described above (all components listed are in weight percent), so as to compare compositions containing organic fluorescent pigment L-88 (instances E-1 and E-2 ), CIELAB parameters of compositions containing phosphorescent pigment QBK58 / UF-A2 (Examples CE-1, CE-2 and CE-3) or compositions without fluorescent pigments (Examples CE-4 and CE-5) and Contrast (a measure of opacity). L-88 is an organic fluorescent pigment, which is a hydroxyl-substituted aryl guanamine. QBK58 / UF-A2 is an inorganic luminescent pigment material, which is made of Y with cerium doping 2 SiO 5 be made of.
[0149] Table 1: Paste formulations of examples E-1 and E-2 and comparative examples CE-1 to CE-5 (content in wt-% provided) .
[0150] components
[0151] The CIELAB parameters and contrast of the formulation above were measured using...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com