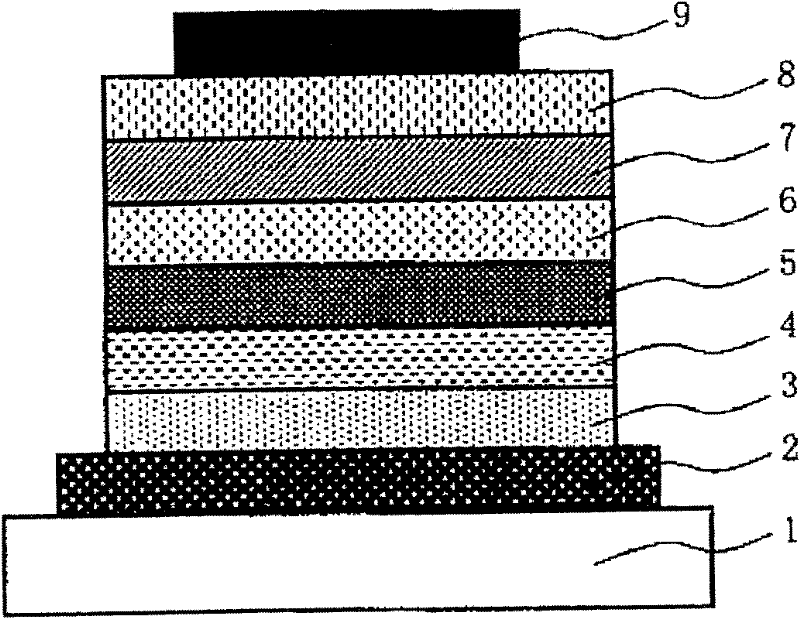
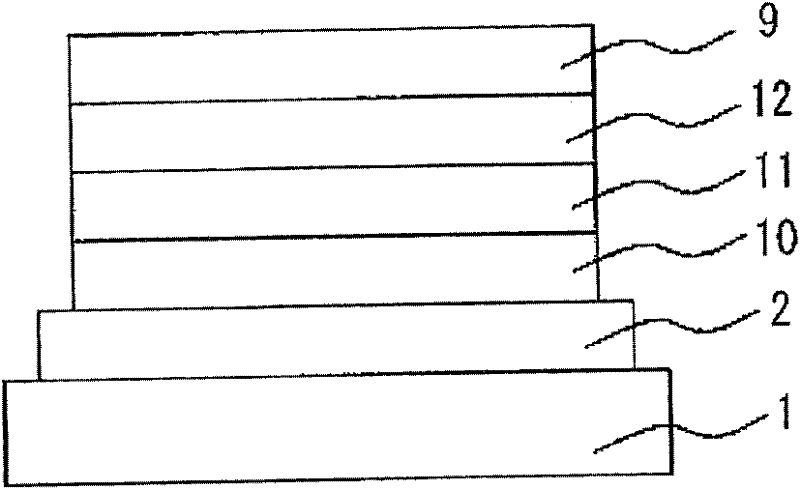
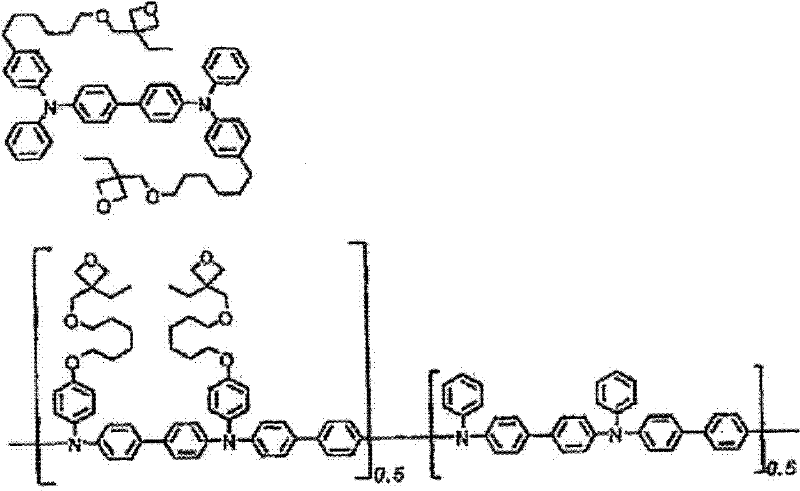
Low-molecular compound, polymer, material for electronic devices, composition for electronic devices, organic electroluminescent element, organic solar cell element, display and lighting equipment
A technology of electronic devices and compounds, applied in the field of low-molecular compounds, which can solve the problems of reduced charge transfer efficiency, reduced luminous efficiency of light-emitting elements, and reduced driving stability, and achieves long driving life, excellent film formation, and low driving voltage. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0674] The present invention will be described in more detail with reference to examples, but the present invention is not limited to the description of the following examples within the range not exceeding the gist of the present invention.
Synthetic example 1
[0676] (Synthesis Example 1) Synthesis of Compound DNOTf
[0677] [chemical formula 39]
[0678]
[0679] In a nitrogen stream at -78°C, under stirring, LiDIAMIN (4.15 g) was added to a mixed solution of DNDION (1.0 g) (manufactured by Aldrich) and tetrahydrofuran (20 ml), and after stirring for 1 hour, N-(2- pyridyl) trifluoromethanesulfonimide (7.88g), stirred at room temperature for 2 days. After adding 50ml of cold water to the reaction solution, carry out ether extraction, then wash the ether solution with water, use MgSO 4 It was dried, concentrated with a rotary evaporator, and purified with a silica gel column to obtain 1.2 g of DNOTf with a purity of 80% by weight.
[0680] (NMR measurement result)
[0681] Compound DNOTf: 1 H NMR (CDCl 3 , 400MHz) δ (ppm) 1.6-1.9 (m, 4H), 3.6-3.8 (m, 2H), 6.1-6.2 (s, 2H)
Synthetic example 2
[0682] (Synthesis Example 2) Synthesis of Compounds BiNpANDN and NpANDN
[0683] [chemical formula 40]
[0684]
[0685] Add ethanol (3g), NpANBOR (0.6g), DNOTf (0.415g), sodium carbonate (1.19g) and water (6g) to toluene (10g) through nitrogen bubbling for 10 minutes, after stirring for 30 minutes, add four (Triphenylphosphine)palladium (0.065 g) was dissolved and stirred at 70°C for 5 hours. The solution was returned to room temperature, 100 ml of water was added, and the precipitated crystals were collected by filtration and washed with ethanol. Then, purification was carried out using recycle separation HPLCLC-9204 (separation column JAIGEL-1H-40) manufactured by Nippon Analytical Industry Co., Ltd. to obtain compound BiNpANDN (0.2 g) and compound NpANDN (0.1 g).
[0686] (Mass measurement result)
[0687] Mass analysis was performed using a mass spectrometer JMS-700 / MStation manufactured by JEOL Ltd. (accelerating voltage: 70eV, emission current change rate: 0A-0.9A...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com