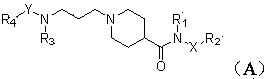
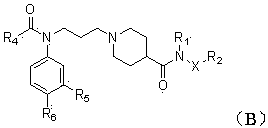
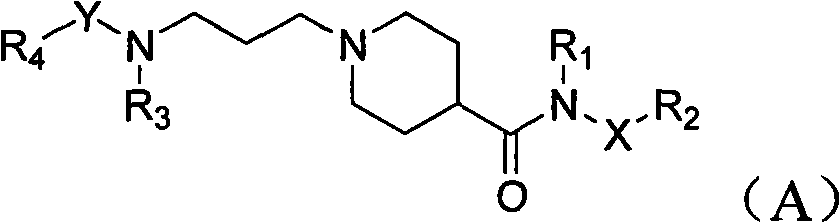
Piperidyl-4-carboxyl amide derivative and preparation method as well as application thereof
A technology for carboxamides and derivatives, which is applied in the field of preparation of 1-piperidine-4-carboxamide derivatives or their pharmaceutically acceptable salts, and can solve the problem of infection of the immune system, drug resistance, low oral availability, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 3-chloro-N-(3-chloropropyl)-benzamide (II)
[0047] Add 1.27g (10mmol) of compound I, 1.57g (10mmol) of 1,3-bromochloropropane, 1.63g (12mmol) of potassium carbonate, and 20ml of acetonitrile into the reaction flask, and reflux for 8-15 hours. After the reaction was completed, it was filtered, and the solvent was recovered under reduced pressure to obtain a crude product, which was separated by column chromatography (eluent: ethyl acetate:petroleum ether=1:10) to obtain 1.2 g of yellow oily liquid, yield: 60%.
[0048] 1 HNMR (δ, CDCl 3 ): 7.34-7.20 (m, 2H, Ar-H), 7.18-7.00 (m, 2H, Ar-H), 3.67-3.64 (m, 2H, CH 2 ), 3.57-3.53 (m, 2H, CH 2 ), 3.05-2.98 (m, 2H, CH 2 ).ESI-MS m / z: 204[M+H] +
Embodiment 2
[0049] Example 2 1-acetyl-N-(3-chlorophenyl)-N-(3-chloropropyl)piperidine-4-amide (IIIa)
[0050] Add 1.02g (5mmol) of compound II, 0.95g (5mmol) of 1-acetylpiperidine-4-acyl chloride, 1mL (6.9mmol) of triethylamine, and 15ml of dichloromethane into the reaction flask, stir at room temperature, and react 4- 6 hours. After the reaction was completed, the solvent was recovered under reduced pressure to obtain a crude product, which was separated by column chromatography (eluent: ethyl acetate: ethanol = 10: 1) to obtain 1.52 g of a tan solid, yield: 85%. M.p. 115-117°C.
[0051] 1 HNMR (δ, CDCl 3 ): 7.31-6.98 (m, 4H, Ar-H), 4.55-4.50 (m, 1H, piperidine-H), 3.83-3.80 (m, 1H, piperidine-H), 3.76 (t, J=7.0Hz, 2H, CH 2 ), 3.54(t, J=7.0Hz, 2H, CH 2 ), 2.94-2.81 (m, 1H, piperidine-H), 2.44-2.34 (m, 2H, piperidine-H), 2.06 (s, 3H, CH 3 ), 2.10-2.06 (m, 2H, CH 2 ), 1.87-1.62 (m, 4H, piperidine-H).ESI-MS m / z: 357[M+H] + .
Embodiment 3
[0052] Example 3 N-(3-chlorophenyl)-N-(3-chloropropyl)-4-fluorobenzamide (IIIb)
[0053] The procedure was the same as in Example 2, except that 1-acetylpiperidine-4-yl chloride was replaced by p-fluorobenzoyl chloride. A tan solid was obtained, yield: 90%. M.p.85-88°C.
[0054] 1 HNMR (δ, CDCl 3 ): 7.27-7.08(m, 4H, Ar-H), 7.03-6.93(m, 2H, Ar-H), 6.89-6.75(m, 2H, Ar-H), 4.04(t, .J=7.0Hz ,2H,CH 2 ), 3.65(t, J=7.0Hz, 2H, CH 2 ), 2.17-2.10 (m, 2H, CH 2 ).ESI-MS m / z: 326[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com