Use of phenol-soluble modulins for vaccine development
A technology for regulating protein and solubility, applied in the field of immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
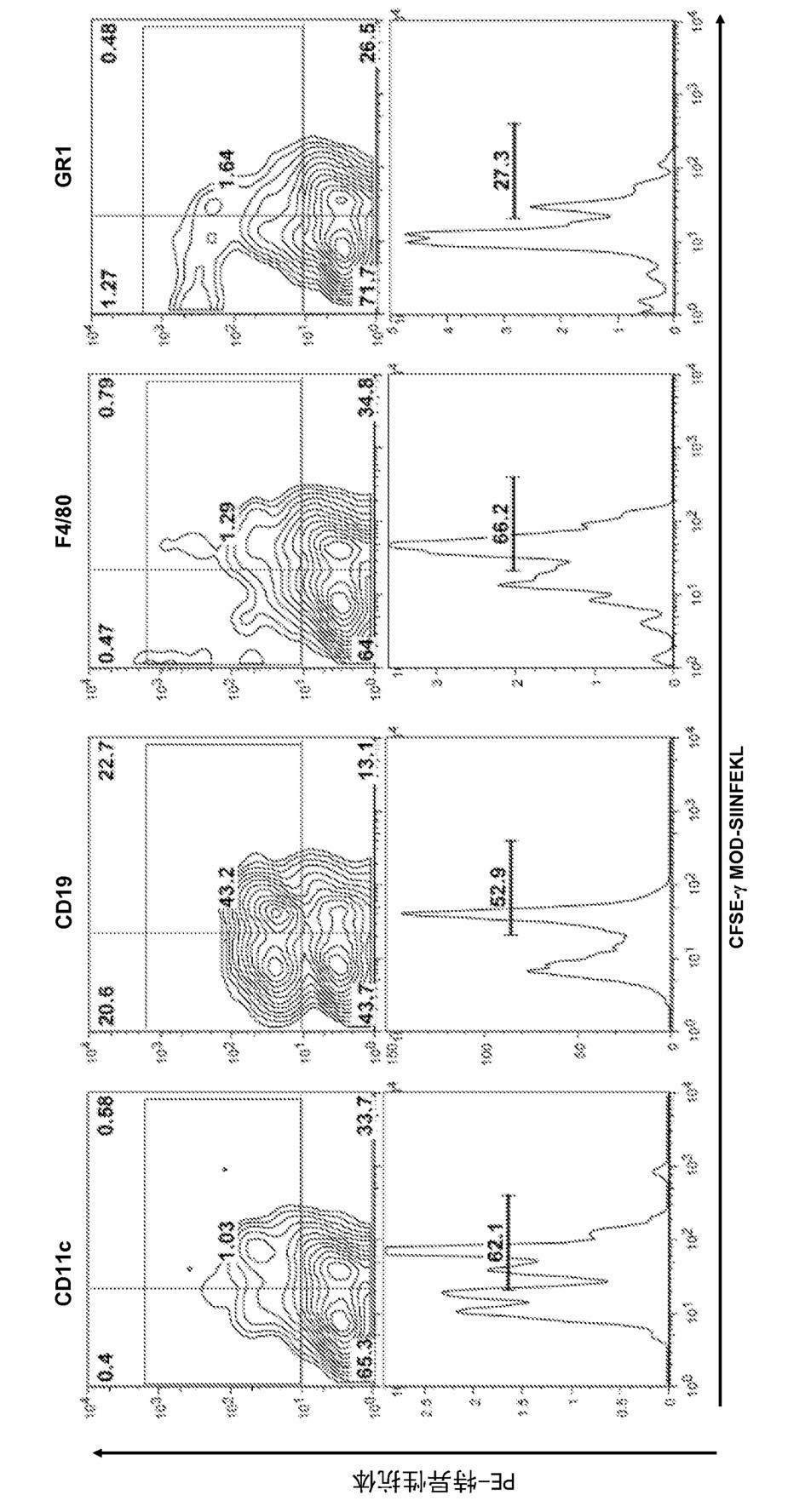
[0285] Example 1 Regulin peptides can bind to the cell surface of splenocytes. binding to regulatory proteins Subpopulations of white-derived peptides were analyzed.
[0286] Materials and methods
[0287] The peptides used in this experiment are listed in Table 3.
[0288] peptide
sequence
SEQ ID NO:
SIINFEKL
SIINFEKL
44
αMod
MADVIAKIVEIVKGLIDQFTQK
1
αMod-SIINFEKL
MADVIAKIVEIVKGLIDQFTQKSIINFEKL
45
SIINFEKL-αMod
SIINFEKLMADVIAKIVEIVKGLIDQFTQK
46
γMod
MAADIISTIGDLVKWIIDTVNKFKK
13
γMod-SIINFEKL
MAADIISTIGDLVKWIIDTVNKFKKSIINFEKL
47
SIINFEKL-γMod
SIINFEKLMAADIISTIGDLVKWIIDTVNKFKK
48
OVA (235-264)
ASGTMSMLVLLPDEVSGLEQLESIINFEKL
49
CFSE-γMod-SIINFEKL
CFSE-MAADIISTIGDLVKWIIDTVNKFKKSIINFEKL
47
CFSE-OVA(235-264)
CFSE-ASGTMSMLVLLPDEVSGLEQLESIINFEKL
49
1073
CVNGVCWTV
50
αMod-1073
...
Embodiment 2
[0295] Example 2 α- Regulin and γ-regulin activate dendritic cell maturation and antigen presentation Materials and methods
[0296] Generation of dendritic cells from bone marrow
[0297] Dendritic cells are grown from bone marrow cells. After lysing erythrocytes with ACK lysis reagent, the cells were washed and incubated with anti-CD4 (GK1; ATCC, Manassas, VA), CD8 (53.6.72; ATCC), Ly-6G / Gr1 (BD-Pharmingen; San Diego CA) and The CD45R / B220 (BD-Pharmingen) antibody mixture was incubated together to remove lymphocytes and granulocytes, followed by addition of rabbit-derived supplements. The remaining cells were grown in 12 Petri dishes using complete medium with 10 6 Cells / ml were supplemented with 20ng / ml mGM-CSF and 20ng / ml mIL-4 (both purchased from Peprotech; London, GB). Replace with fresh cytokine-containing medium every 2 days. On day 7, non-adherent dendritic cells were harvested and cultured in the presence or absence of 50 μM Modulin peptide or 1 μg / ml LPS ...
Embodiment 3
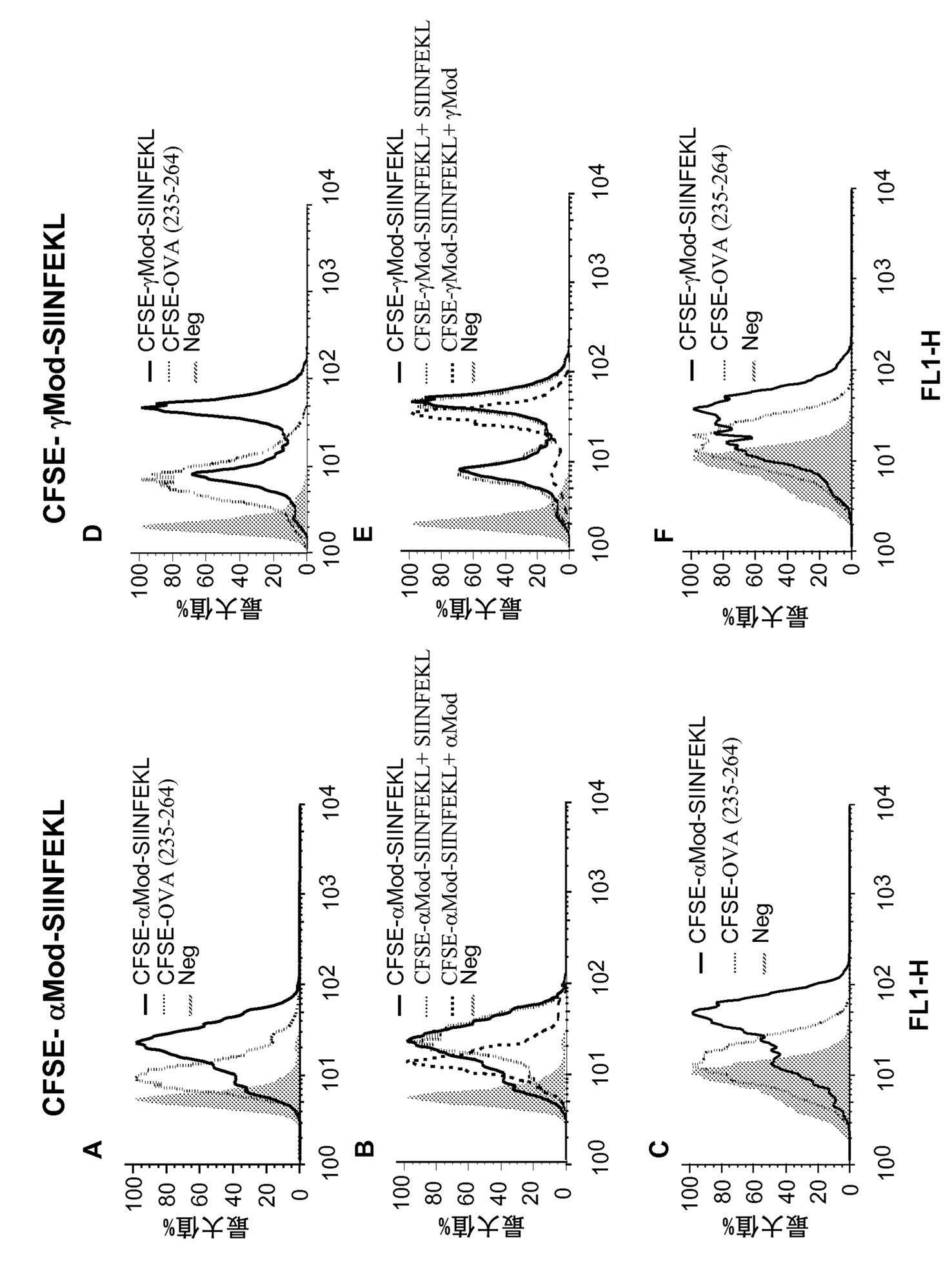
[0308] Example 3A - using a regulatory protein-derived peptide that binds to the cytotoxic SIINFEKL antigen with some Co-immunization with TLR ligands induces activation of cytotoxic responses to antigens. The cytotoxicity should The answer was long-lasting and protected mice against subcutaneous injection of EG7OVA1 tumor cells.
[0309] Materials and methods
[0310] Induction of cytotoxic T lymphocytes (CTL) in vivo and quantification of IFN-γ-producing cells after immunization (ELISPOT).
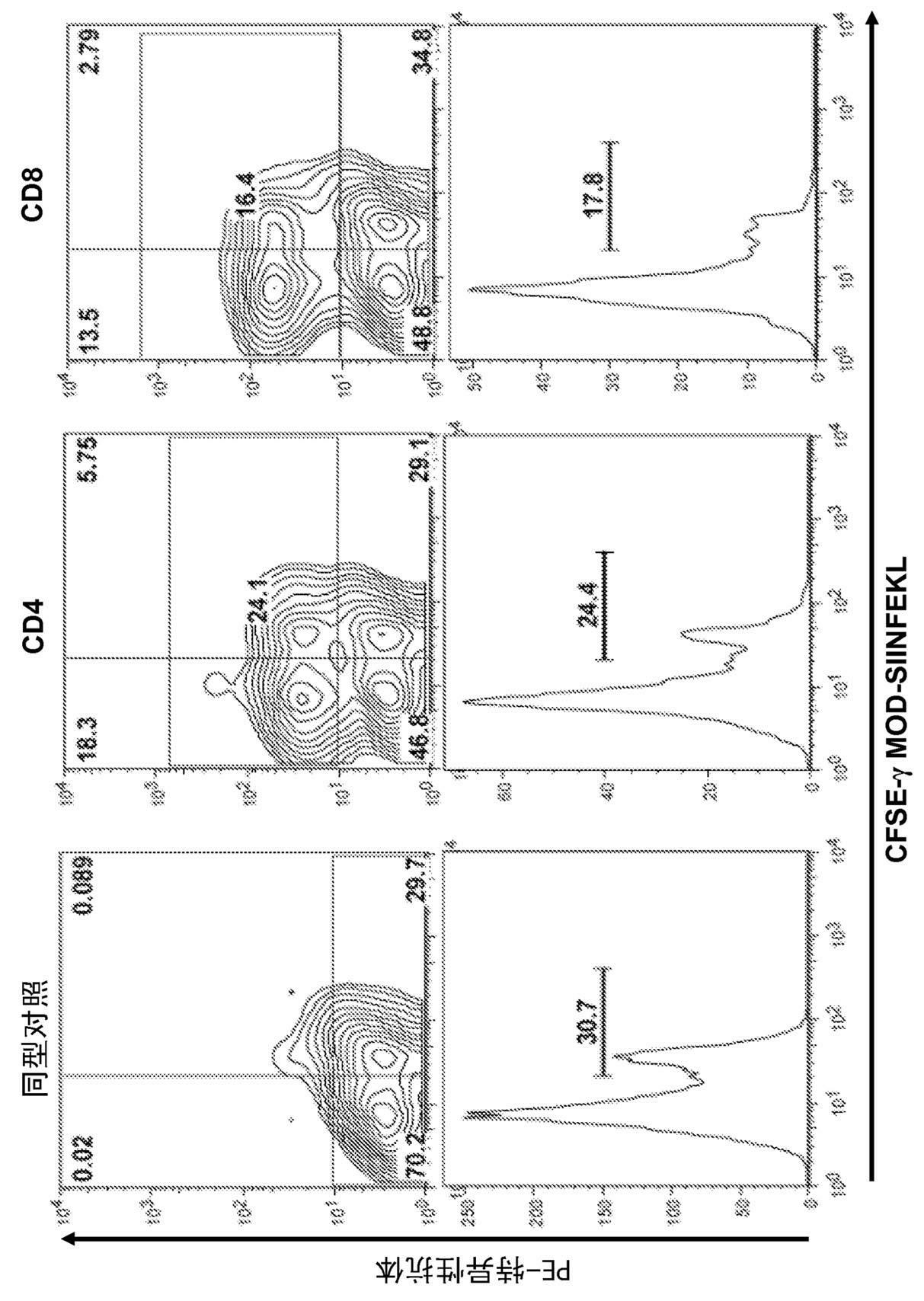
[0311] C57BL6 mice were immunized intravenously with 5nmol of the indicated peptide and 50μg of TLR3 ligand polymyocytes. In some cases, peptide immunization was performed in the presence of TLR2 (peptidoglycan), TLR4 (LPS or EDA protein) or TLR9 (CpG) ligands. Seven days after immunization, mice were injected intravenously with 5x10 6 A mixture of splenocytes, typically half of which were previously labeled with a 0.25 μM dose of C and the other half with 2.5 μM CFSE and SIIN...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com