Process for co-producing sulfuric acid, fine iron powder and iron oxide red by ferrous sulfate heptahydrate and pyrite
A ferrous sulfate, pyrite technology, applied in the direction of iron oxide/iron hydroxide, iron oxide, sulfur compounds, etc., can solve the problem of slow decomposition speed of ferrous sulfate, high energy consumption in the production process, incomplete decomposition of ferrous sulfate and other problems, to achieve the effect of simplifying the production process and equipment investment, increasing the added value of products, and improving the value of use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
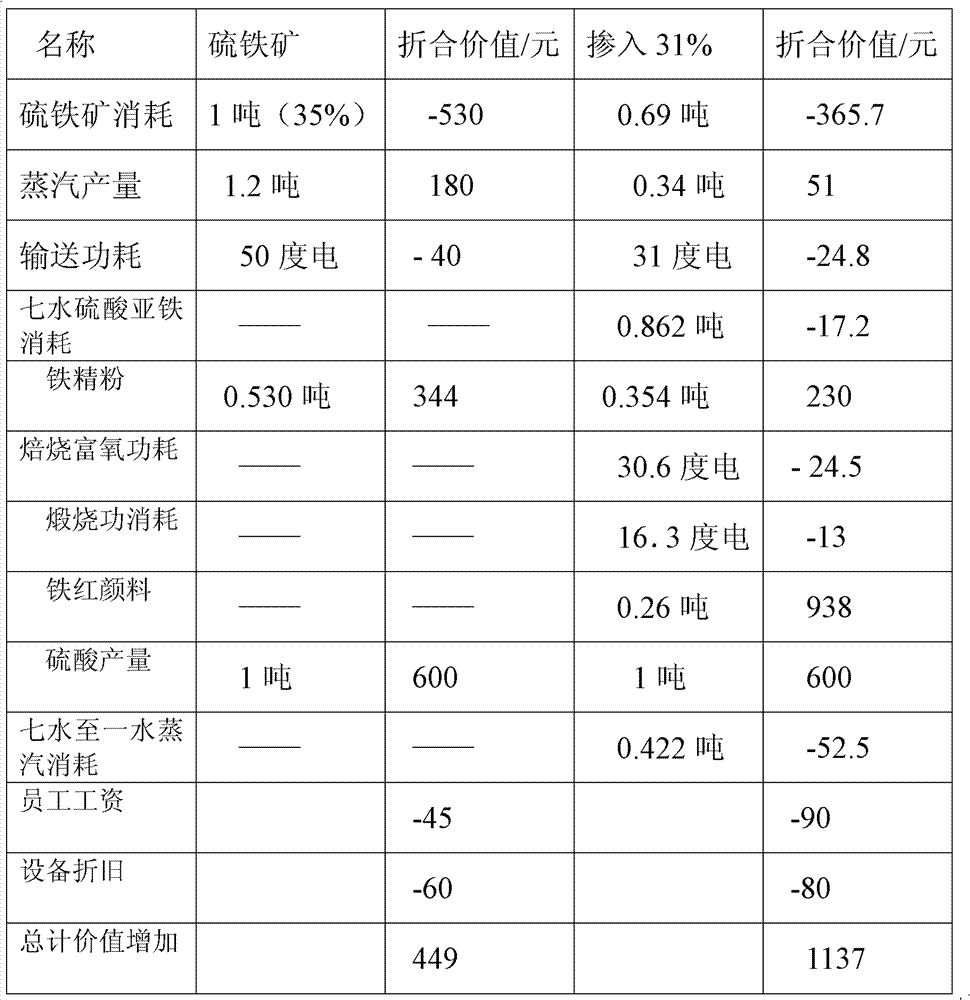
[0113] The example uses the production capacity of 300,000 tons of sulfuric acid as a specific illustration
[0114] 1. The sulfuric acid output is 300,000 tons, and the sulfuric acid output per hour is 37.5 tons, wherein ferrous sulfate heptahydrate accounts for 31% of the acid production, and the consumption of 95% ferrous sulfate heptahydrate is 34 tons / hour; The iron ore burning rate is 99%, the sulfur content is 46%, and the consumption of pyrite with a moisture content of 2% is 27.15 tons / hour.
[0115] 2. The heat required to convert ferrous sulfate heptahydrate into ferrous sulfate monohydrate is 8.1×10 6 kcal / h, the heat loss in the volatilization process of steam drying or heat treatment is 10%, the heat release in the steam heating process is calculated as 563kcal / kg, the steam consumption is 15.8t / h, and the steam consumption converted into a ton of acid is 0.422t / t sulfuric acid.
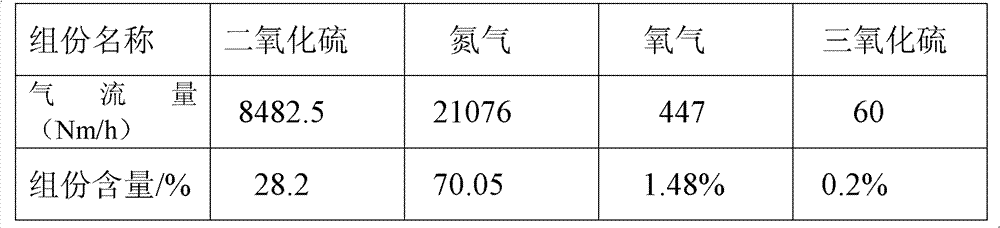
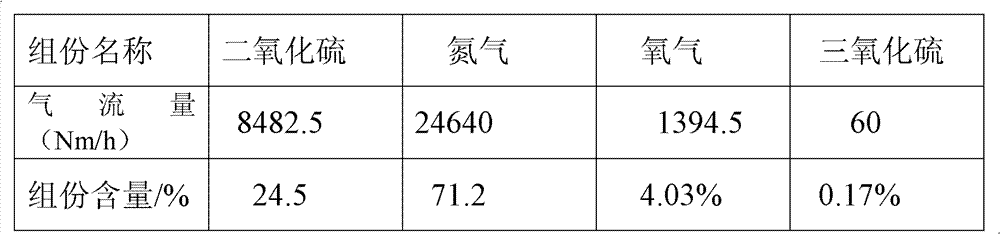
[0116] 3. Mix the formed ferrous sulfate monohydrate and pyrite into the ore hopp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com