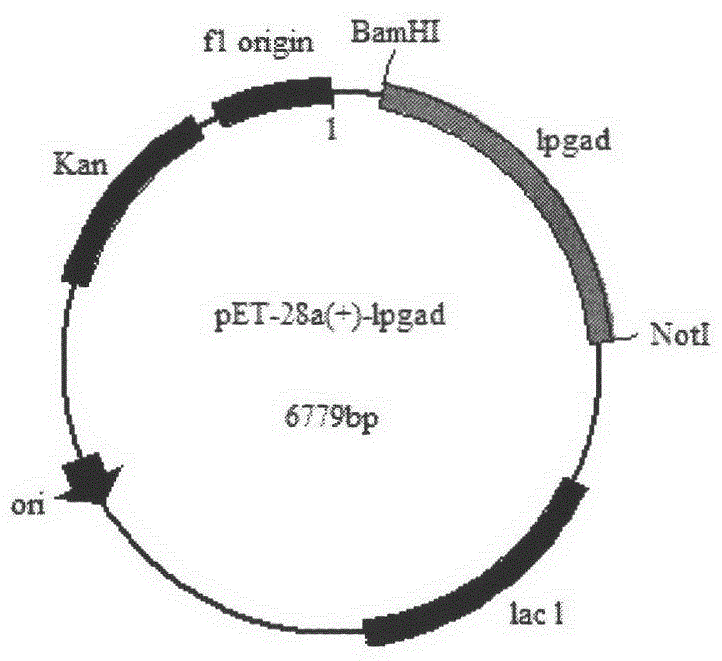
A construction method and application of high-yielding γ-aminobutyric acid recombinant E. coli/pet-28a-lpgad
A pet-28a-lpgad and pmd18-t-lpgad technology, applied in the biological field of fermentation engineering, can solve the problems of GABA production efficiency, pollution, long production cycle, etc., and achieve strong technology portability, easy promotion and application, The effect of short conversion cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: Construction of recombinant Escherichia coli BL21 / pET-28a-lpgad
[0026] According to the lpgad gene sequence in the whole genome nucleic acid sequence 3254376bp of Lactobacillus plantarum subsp.plantarum ST-III (GI: 308044682) in NCBI, the primers of the glutamic acid decarboxylase coding gene were designed:
[0027] P1: GAC (GGATCC) ATGGACCAGAAGCTGTTAAC (BamH I)
[0028] P2: GGC(GCGGCCGC)TCAGGTGTGTTTAAAAGCTGTT(Not I)
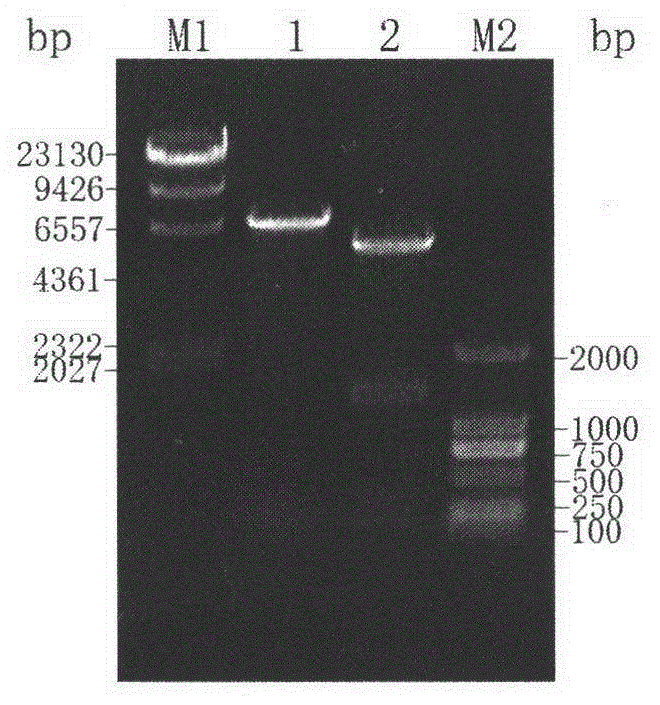
[0029]The total Lactobacillus plantarum chromosome was extracted as a template, and the target gene fragment was obtained by PCR amplification. The PCR amplification conditions were 94°C, 5min pre-denaturation; 94°C for 50s, 57°C for 1min 30s, 72°C for 2min, 35 cycles; 72°C for 10min. The resulting fragment was recovered by gel and ligated with the cloning vector pMD18-T, transformed into E.coli JM109, and screened on an ampicillin resistance plate to pick out positive transformants. The extracted plasmid was digested and identified, and...
Embodiment 2
[0030] Embodiment 2: Expression and Ni-NTA purification of recombinant glutamic acid decarboxylase
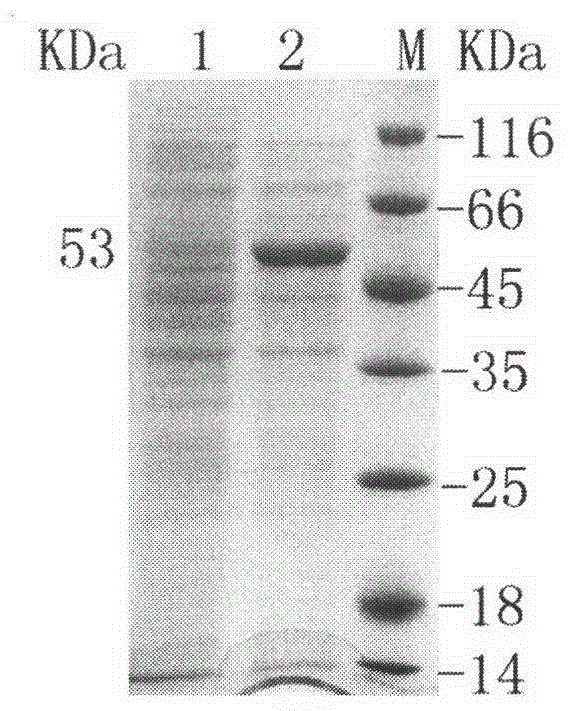
[0031] Inoculate the recombinants stored in cryopreserved tubes into LB medium containing kanamycin (final concentration: 50 μg / mL), culture overnight at 37°C with shaking, transfer at 1% inoculum size the next day, and culture at 37°C until OD approx. 0.6-0.8, add human IPTG to a final concentration of 0.5mmol / L, and induce expression overnight at 16°C. The cells induced by IPTG were ultrasonically disrupted, and the supernatant was analyzed by SDS-PAGE, and a specific band with a molecular weight of about 53kDa was detected, as shown in image 3 shown. The specific enzyme activity measured in the supernatant was 8.53U / mg.
[0032] Centrifuge the overnight induced expression bacterial solution at 10000r / min, 4°C for 15min, collect the bacterial cells, suspend the bacterial cells with pH 7.4 PBS buffer solution, ultrasonically break the cells, and then filter through a 0.45 μ...
Embodiment 3
[0033] Example 3: Preliminary Study on the Enzymatic Properties of Recombinant Glutamic Acid Decarboxylase
[0034] 1) Optimum pH and pH stability: Prepare acetic acid-sodium acetate reaction buffer with pH 3.6-6.0, mix the enzyme solution with different pH reaction solutions at 30°C, and measure the enzyme activity of GAD in different pH reaction systems To investigate the effect of pH on enzymatic reactions. Then add a certain amount of enzyme solution to the above reaction buffers with different pH, and keep them warm at 30°C for 2 hours, measure the remaining enzyme activity, and investigate the stability of GAD under different pH conditions.
[0035] 2) Optimum temperature and thermal stability: Use pH4.8 reaction buffer solution at 20°C, 25°C, 30°C, 35°C, 37°C, 40°C, 45°C, 50°C, 55°C, 60°C , 65 ℃, 70 ℃ to measure the enzyme activity, study the impact of temperature on the enzyme reaction. The enzyme solution was incubated at the above different temperatures for 2h, 4h,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com