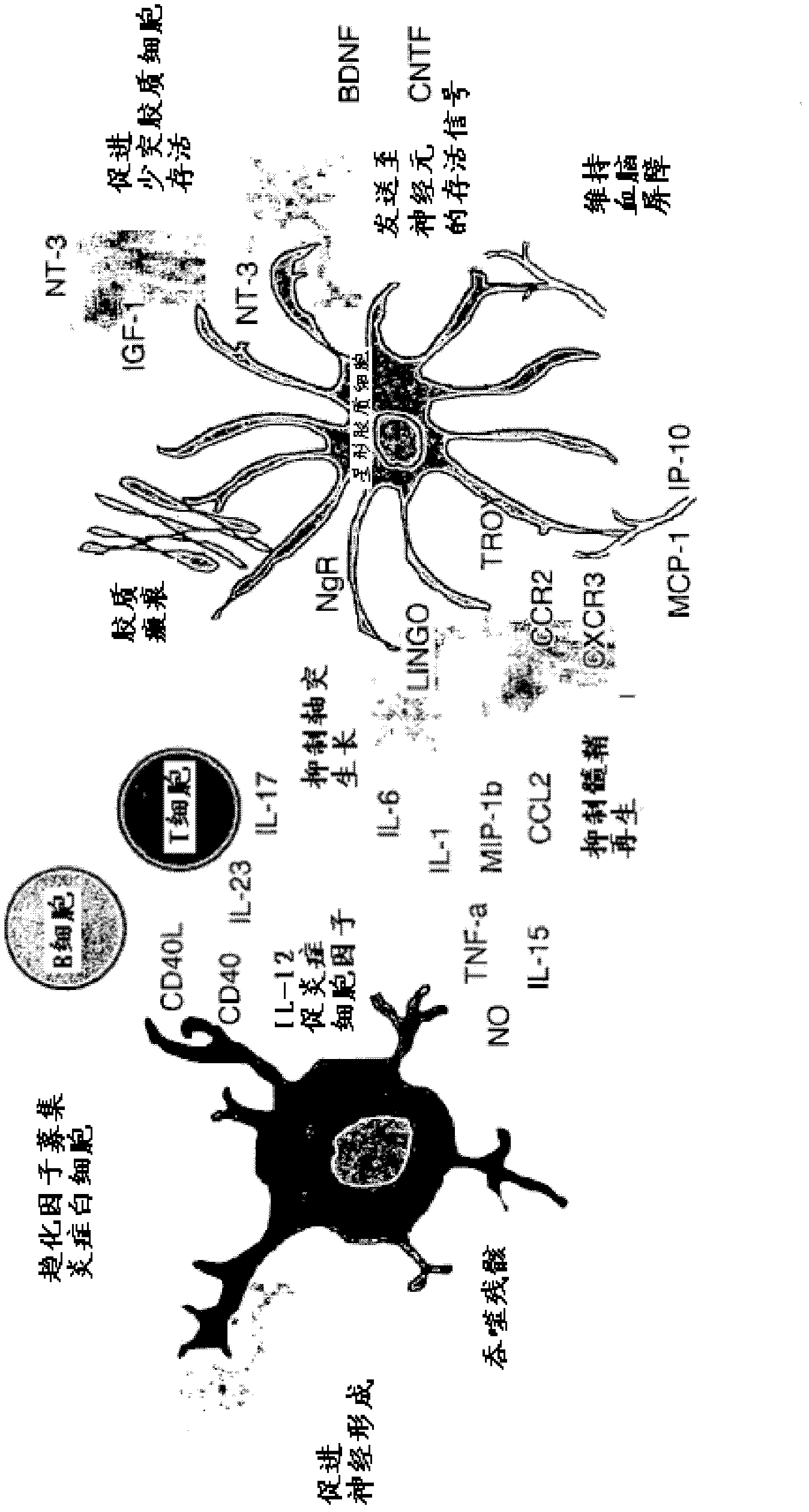
Treatment of neurodegeneration and neuroinflammation
A technology for neuroinflammation and neurodegeneration, which is applied in the field of neurodegeneration and neuroinflammation treatment, and can solve problems such as new recurrence and increased stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
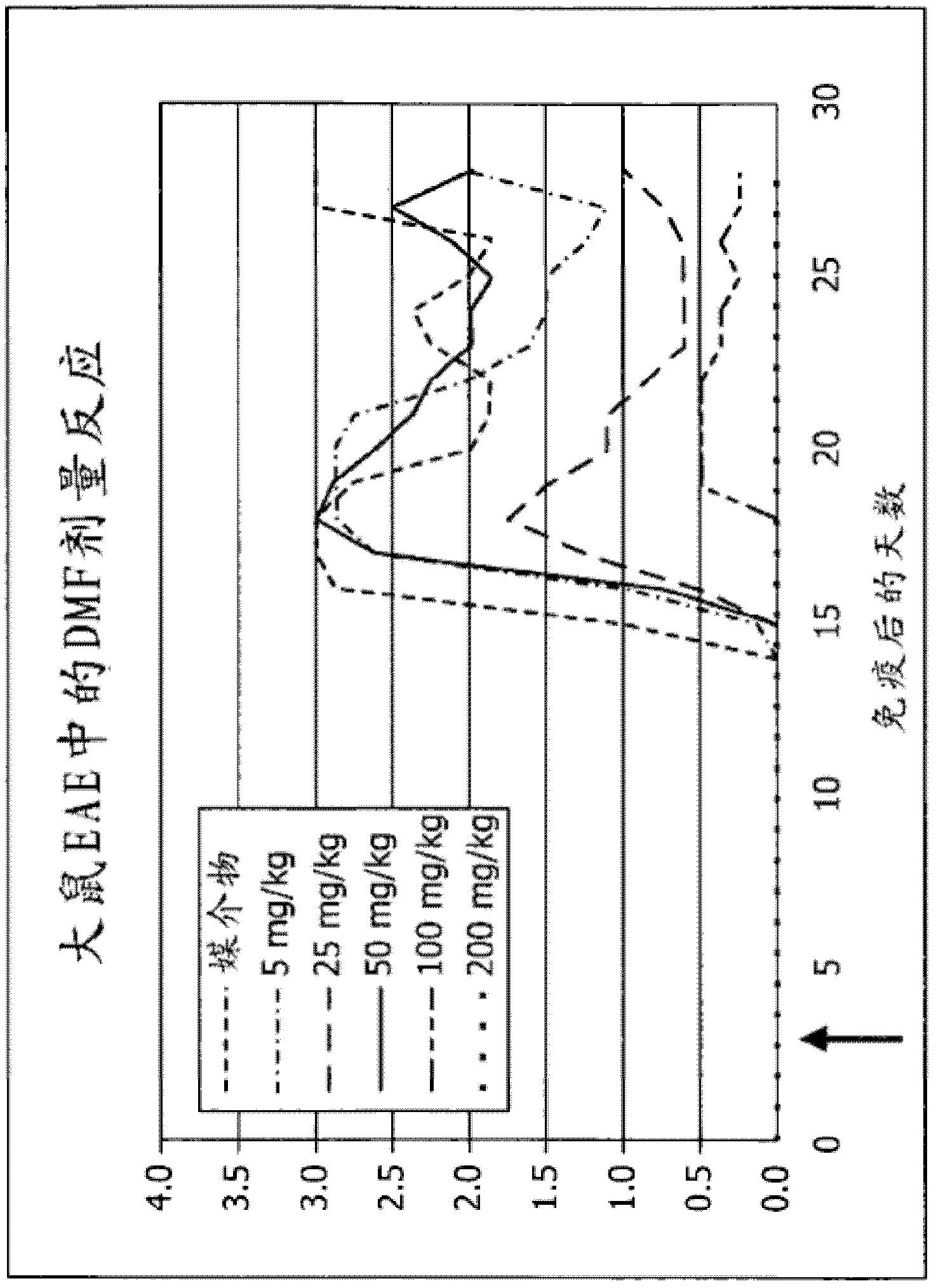
[0094] BG00012, an orally available formulation of dimethyl fumarate (DMF), is in Phase III testing for relapsing-remitting multiple sclerosis (RRMS). In phase IIb testing, BG-12 significantly reduced gadolinium-enhancing brain lesions and reduced T1 hypointense black holes. The active ingredient of BG00012, dimethyl fumarate (DMF), was tested in a rat model of EAE. Such as Figure 1A As shown, treatment of experimentally induced EAE rats with various doses of DMF alleviated EAE symptoms in a dose-dependent manner. Treatment with 200 mg / kg DMF completely eliminated the disease. ( Figure 1A ).
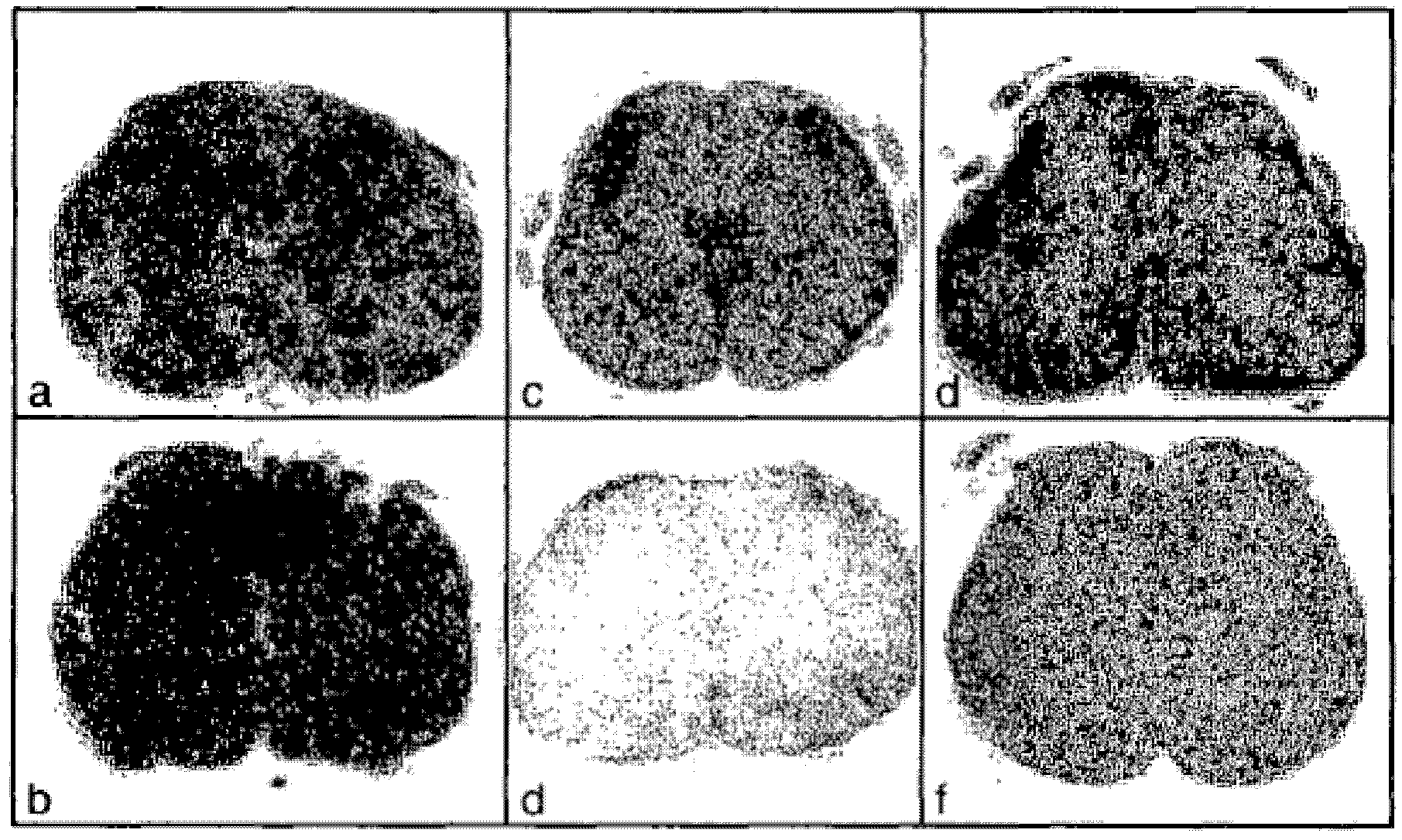
[0095] Figure 1B Cross-sections of spinal cords of rats treated with vehicle or BG00012 are shown. Panels a–f were processed and stained as follows:
[0096] a.Luxol Fast Blue / vehicle
[0097]b.Luxol Fast Blue / BG00012
[0098] c. GFAP / vehicle
[0099] d. GFAP / BG00012
[0100] e.IBA1 / vehicle
[0101] f.IBA1 / BG00012
[0102] Such as Figure 1B As shown, the activated astro...
Embodiment 2
[0104] This example shows that BG00012 reduces astrocyte activation in vivo. Specifically, there were fewer activated astrocytes in the gray matter of the spinal cords of DMF-treated rats than in the spinal cords of rats that received vehicle alone. Figure 3A Shown is a cross-section of the spinal cord stained with GFAP antibody to identify activated astrocytes. Panels (a) and (b) are from rats treated with vehicle only at 5x and 20x magnification, respectively, while panels (c) and (d) are from rats treated with 100 mg / kg DMF at 5x magnification, respectively and 20 times the display.
[0105] Figure 3B Morphometric quantification using Apeiro color deconvolution is shown.
[0106] Figure 3C A cross-section of the rat spinal cord is shown, with ventral gray and white matter areas indicated. These regions were selected for morphometric assays. Figure 3D Morphometric quantification of positive GFAP staining in ventral gray and white matter is shown.
Embodiment 3
[0108] This experiment shows that BG00012 reduces the activation of primary cultured astrocytes. Figure 4A Quantitative PCR analysis of GFAP expression is shown after 6 and 24 hours of stimulation with the indicated concentrations of DMF. As shown, DMF inhibited GFAP expression in a concentration-dependent manner.
[0109] Figure 4B Quantitative PCR analysis of TNF expression is shown after 24 h stimulation with the indicated concentrations of DMF with 0, 10 and 30 ng / ml LPS added 4 h before harvest. As shown, LPS induced TNF expression in a dose-dependent manner, and the induced TNF expression was inhibited by DMF in a dose-dependent manner.
[0110] Figure 4C Quantitative PCR analysis of NQO1 expression is shown after 6 and 24 hours of stimulation with the indicated concentrations of DMF. As shown, DMF induced Nqo1 expression in a concentration-dependent manner. It is also evident from the data that Nqo1 is induced at a higher level by 24 hours exposure to DMF than b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com