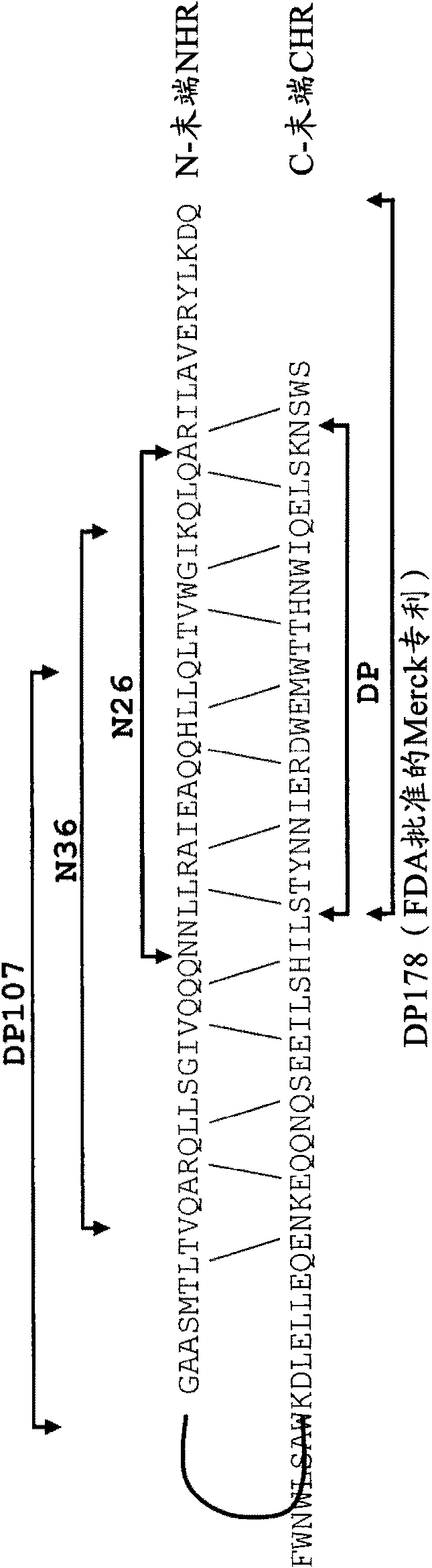
Lipopeptide inhibitors of HIV-1
A lipophilic, fatty acid-based technology applied in the field of HIV-1 lipopeptide inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0197] Example 1: Anchoring of N36 to membranes enhances its inhibitory activity:
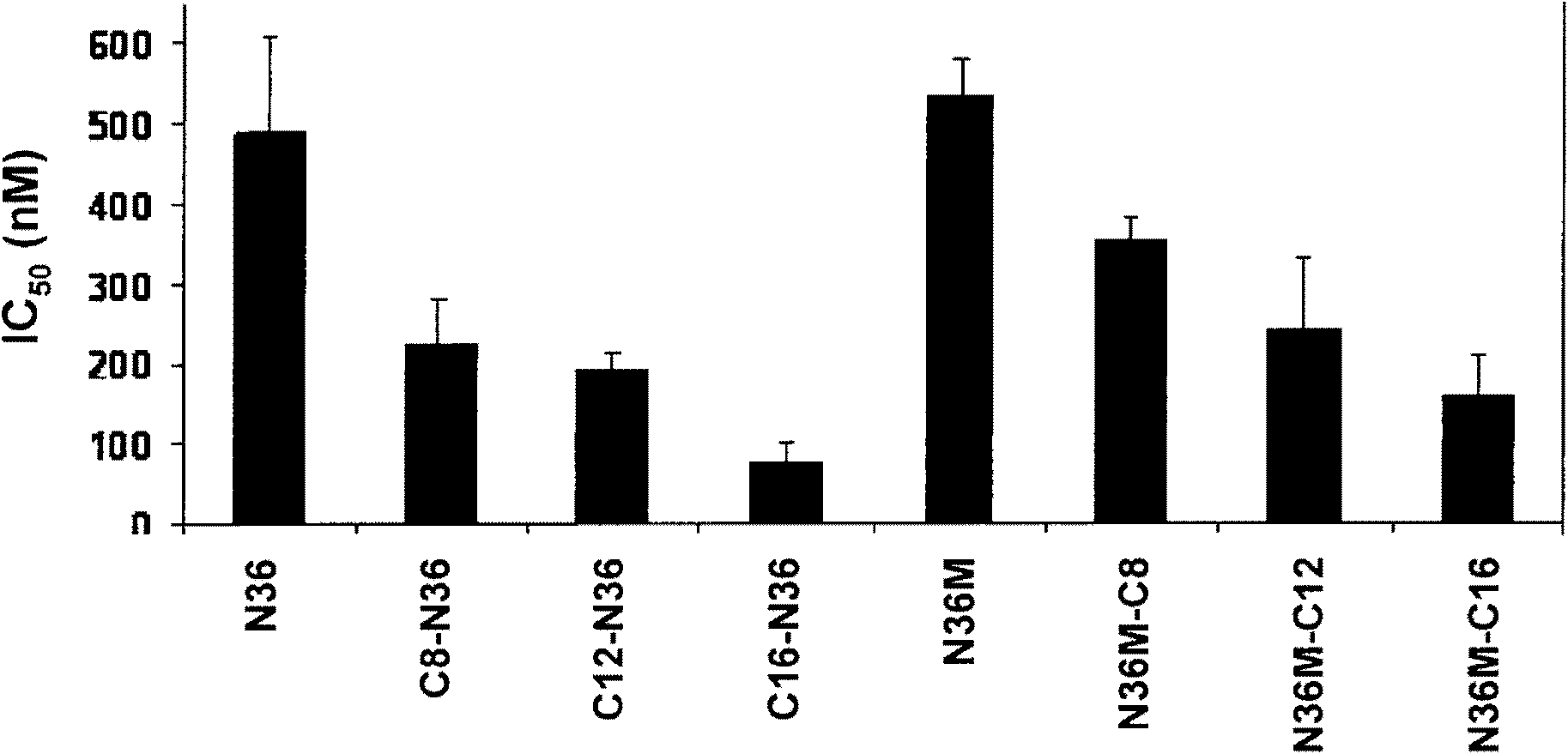
[0198] To carefully study the role of N36 anchoring to the membrane, we conjugated octanoic acid, dodecanoic acid and palmitic acid to the N-terminus of N36 (Table 1). The resulting peptides C8-N36, C12-N36 and C16-N36 (Table 1) were examined in a cell-cell fusion inhibition assay and the results are shown in figure 1 middle. A correlation was observed between the length of the conjugated fatty acid and the inhibitory activity of the N-conjugated N36 peptide. N36, C8-N36, C12-N36 and C16-N36 exhibited ICs of 488±119nM, 222±56nM, 190±21nM and 72±27nM, respectively 50 value. Interestingly, AcN36 was not active up to 2000 nM; we therefore considered it to be inactive. This compares with ICs of 16000 ± 2000 nM and 584 ± 46 nM indicating the acetylated and non-acetylated forms of N36 50 This is related to previous studies of (Bewley et al., 2002, J. Biol. Chem. 277: 14238-45). Taken together, ...
Embodiment 2
[0201] Example 2: The orientation of anchored N36 to the endogenous CHR region is not critical:
[0202] To examine the importance of proper orientation of the N36 peptide in relation to the prefusion conformation, we also conjugated octanoic, dodecanoic and palmitic acids to the C-terminus of a modified N36, termed N36M (Table 1). The parental peptides and the resulting fatty acid conjugated peptides N36M, N36M-C8, N36M-C12, and N36M-C16 were examined in a cell-cell fusion inhibition assay (Table 1), and the results are in figure 1 available in . Likewise, a correlation was observed between conjugated fatty acid length and C-conjugated N36 peptide. N36M, N36M-C8, N36M-C12 and N36M-C16 exhibited IC50 values of 531±48nM, 354±25nM, 241±89nM and 159±47nM, respectively. Since acetylation of N36 abolishes its activity, we added acetyl groups to N36M-C12 and N36M-C16 to obtain AcN36M-C12 and AcN36M-C16. These two lipopeptides were examined in a cell-cell fusion inhibition assay...
Embodiment 3
[0204] Example 3: Analysis of Inhibition Curves Shows Different Modes of Inhibition for the Peptides of the Invention:
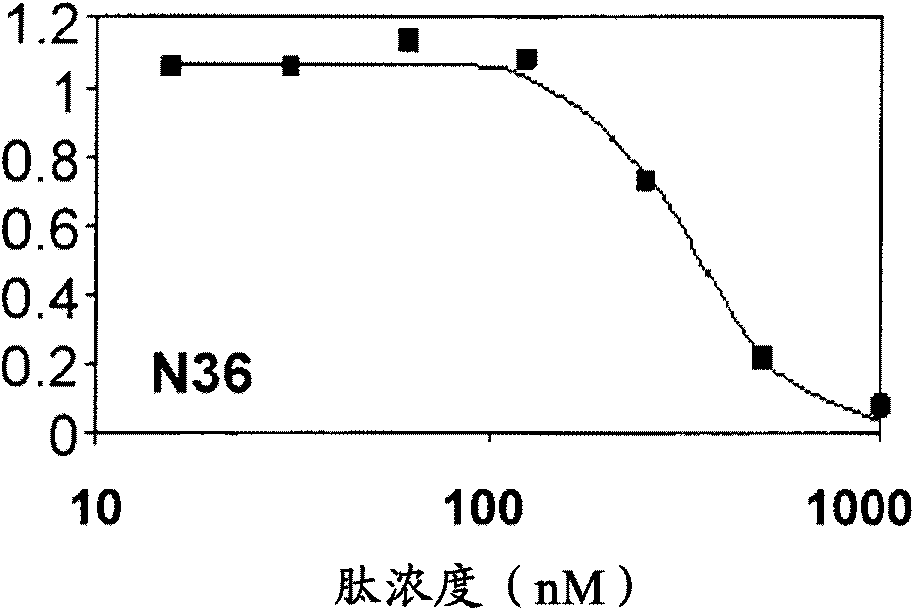
[0205] Figure 3 presents a representative experiment showing the inhibitory activity curves of N36 and its N-terminal fatty acid conjugated analogs. Figure 3 shows the different shapes of the binding curves of different peptides from S-shaped through intermediate shapes to hyperbolic. The S-shape can be explained by the oligomerization propensity of N36. Therefore, without wishing to be bound by theory or mechanism of action, we speculate that the different binding profiles may be due to different inhibitory oligomeric states of the peptides. Therefore, for the best fit, we applied the equation containing the synergy parameter, in this case indicative of the inhibitory oligomeric state of the peptide. Therefore, after the fit is achieved, the c value represents the oligomeric state of the peptide. Figure 4 Values of oligomerization parameters for diffe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com