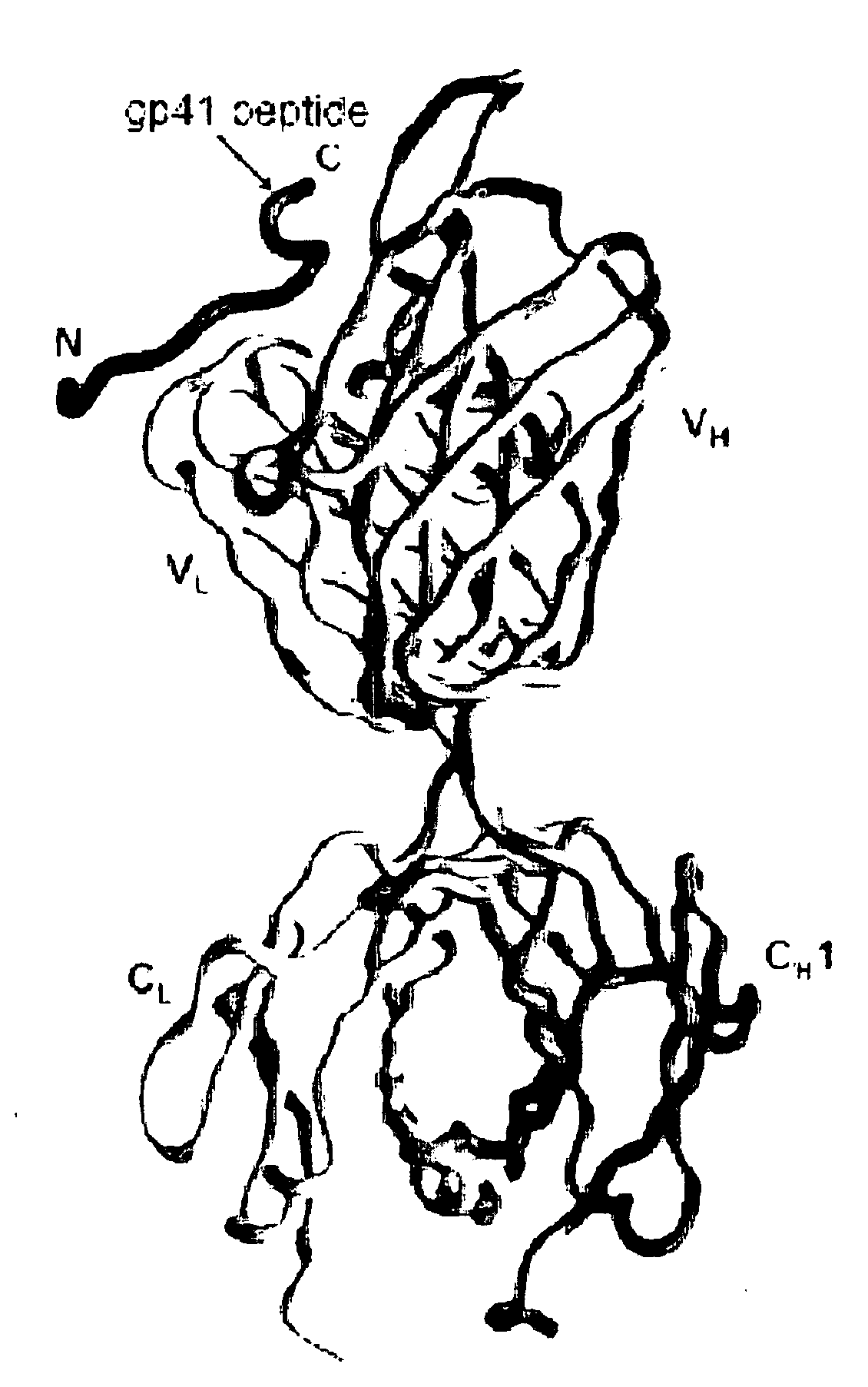HIV vaccine immunogens and immunization strategies to elicit broadly-neutralizing anti-HIV-1 antibodies against the membrane proximal domain of HIV GP41
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Structure and Mechanistic Analysis of the Anti-Human Immunodeficiency Virus Type 1 Antibody 2F5 in Complex with Its gp41 Epitope
2F5 IgG and Fab Production.
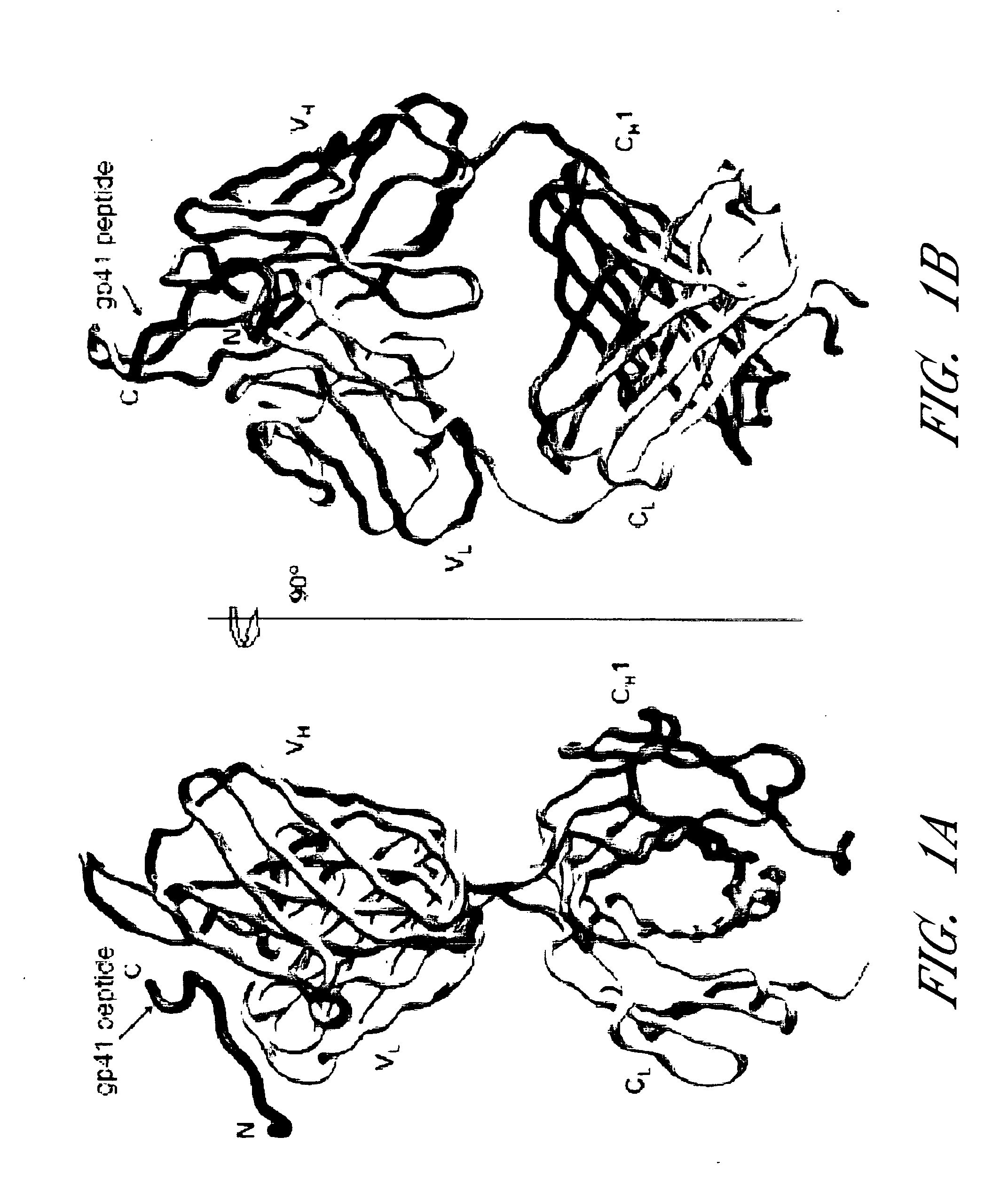
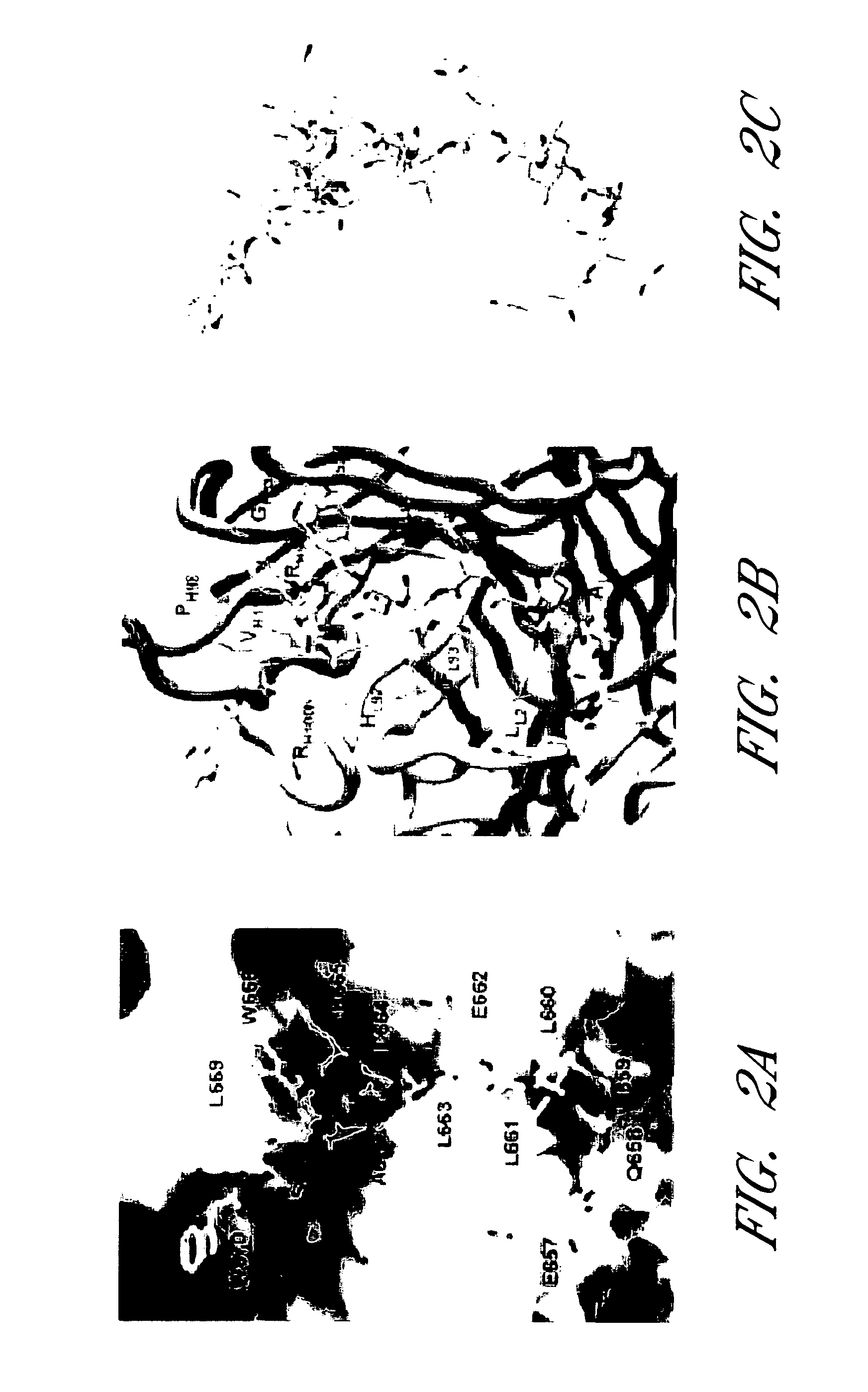
[0055]Human monoclonal antibody 2F5 is produced by recombinant expression in Chinese hamster ovary cells (Kunert et al., 2000, Stable recombinant expression of the anti HIV-1 monoclonal antibody 2F5 after IgG3 / IgG1 subclass switch in CHO cells, Biotechnol. Bioeng. 67:97-103). The 2F5 antigen-binding fragment (Fab) is prepared by reducing 2F5 immunoglobulin G (IgG) (13 mg / ml) in 100 mM dithiothreitol (1 h, 37° C.), alkylating in 2 mM iodoacetamide (48 h, 4° C.), and then cleaving with endoproteinase Lys-C (0.01 μg / μl; Roche Applied Sciences) in 25 mM Tris Cl and 1 mM EDTA (pH 8.5) for 4 h at 37° C. The cleavage reaction is stopped with 1 mM TLCK (Na-p-tosyl-L-lysine chloromethyl ketone) and 0.4 mM leupeptin, and the cleavage products are passed over a protein A-Sepharose column (Sigma). Flow-through fractions are collected and subj...
example 2
Examples of Immunogen Platforms
[0097]Three examples of immunogen platforms are shown in FIG. 10. Panels A and B show schematic illustrations of the results of immunizations with nucleic acid molecules (i.e. genetic immunizations) that encode the 2F5 epitope in association with the 4E 10 & Z13 epitopes, a transmembrane domain, and a heterologous protein X (Panel A) or gp41 ectodomain sequence (Panel B) that have been designed for desired presentation of the 2F5 epitope. FIG. 10, Panel C is a schematic representation of a 2F5 peptide immunogen, wherein the peptide has been designed for the desired presentation of the 2F5 epitope.
Potential gp41 constructs for genetic immunization are outlined below:
“26mer” refers to the membrane proximal region of gp41 from NEQ through the 4E10 epitope to the putative beginning of the transmembrane domain (NEQELLELDKWASLWNWFNITNWLWYIK (SEQ ID NO: 20)
“CD5 leader” refers to the leader sequence of the CD5 scavenger receptor protein.
“C9” refers to short pe...
example 3
Analysis of 2F5 Antibody Sequence Homology with Other Immunoglobulins
[0101]The nucleotide sequence of the heavy chain variable region of the 2F5 antibody is used as query sequence find homologous immunoglobulins. The following immunoglobulins are identified: X69690; L21964; X62111; L21972; L21968. This analysis reveals that the 2F5 antibody has a high degree of sequence homology with autoreactive antibodies, especially with an anti-cardiolipin antibody X69690 (AF455551), which also utilizes the D3-3 D segment germline gene. The sequences of polynucleotides encoding these genes is shown below:
Input(SEQ ID NO: 21)agg atc acg tta aag gaa tcg ggt cct ccg ctg gtgaaa ccc aca cag act ctc acg ctg acc tgt tcc ttctct ggg ttc tca ctg tcc gat ttt gga gtg ggt gtgggc tgg atc cgt cag ccc cca gga aag gcc cta gagtgg ctt gca atc att tat tcg gat gat gat aag cgctac agc cca tcg ctg aac acc aga ctc acc atc accaag gac acc tcc aaa aat caa gtt gtc ctt gtc atgact agg gtg agt cct gtg gac aca gcc acg tat ttctg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com