Preparation and application of curcumin analogs capable of inhibiting activity of 11beta-hydroxysteroid dehydrogenase type II (11beta-HSD2) of human
A technology of hydroxysteroids and dehydrogenase inhibitors, which is applied in the field of medicine and can solve problems such as low bioavailability and poor human absorption efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
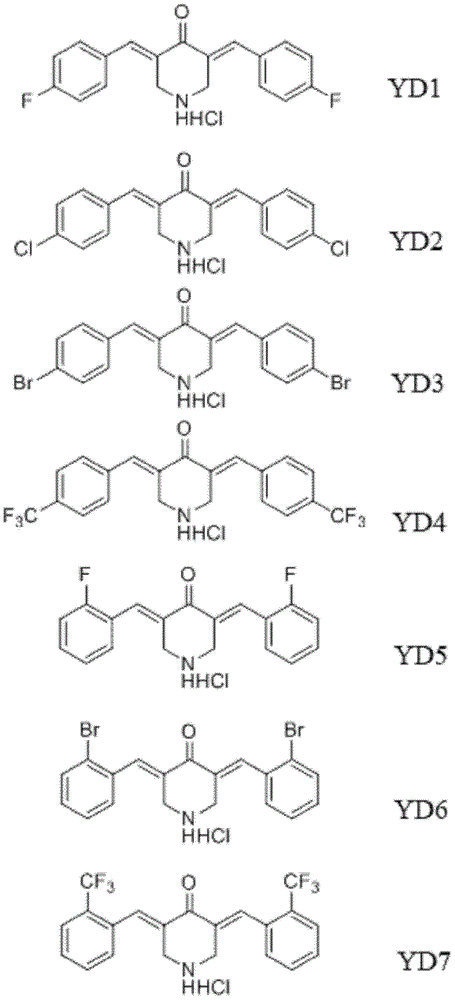
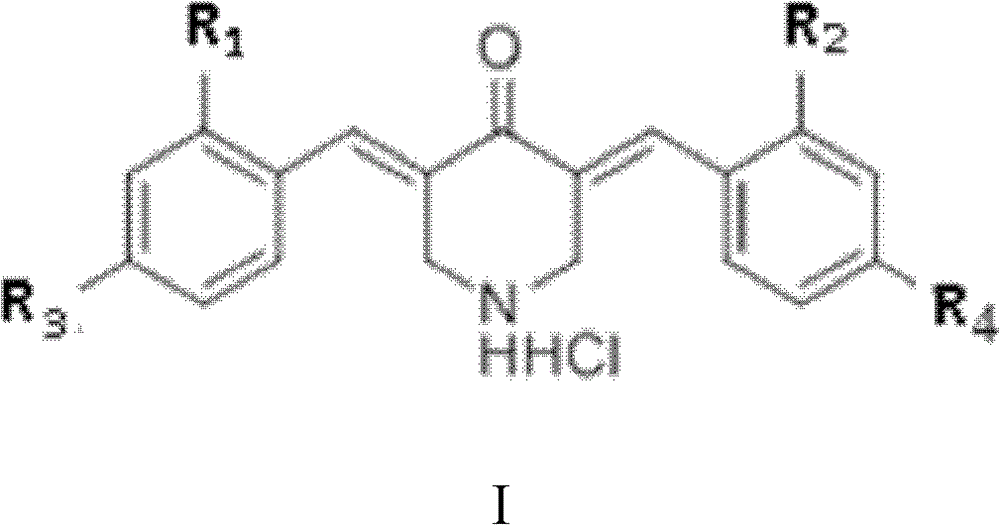
[0055] Example 1: Preparation and identification of 7 curcumin analogues
[0056] (1) 3,5-bis[4-(fluoro)benzylidene]piperidin-4-one, namely YD1
[0057]
[0058] Weigh 1.0g (7.5mM) of piperidone hydrochloride and 1.8g (15mM) of p-fluorobenzaldehyde in a round bottom flask, add 10ml of absolute ethanol to dissolve, then slowly add 5ml of concentrated hydrochloric acid dropwise under ice bath conditions, magnetically Stir and heat to reflux for 6 hours. The separation and purification refer to cooling the filtrate obtained after heating to reflux to room temperature, and simultaneously, a yellow solid is precipitated. After filtering the suspension with a sand core funnel, the solid matter was collected, washed 3 times with absolute ethanol, and vacuum-dried overnight at 30° C. to obtain 1.83 g of a yellow powder product, which was purified by silica gel column chromatography to obtain 98% of the yellow compound. The yield is 65.2%. 1 H NMR (500MHz, DMSO) δ: 10.23(s, 2H), ...
Embodiment 2
[0078] Example 2: Detection of 7 kinds of curcumin analogues inhibiting human kidney cell type 2 11β-hydroxysteroid dehydrogenase activity
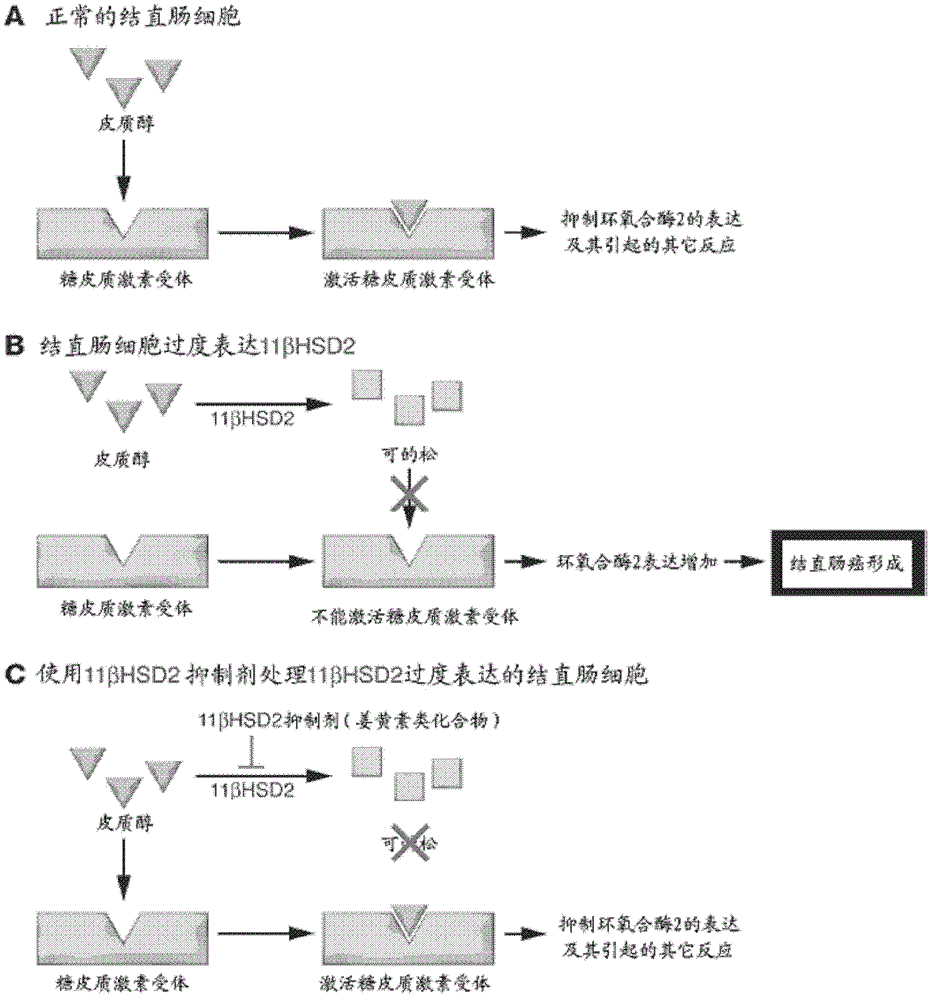
[0079] Type 2 11β-hydroxysteroid dehydrogenase (11β-hydroxysteroid dehydrogenase type II, 11β-HSD2) is an enzyme with nicotinamide adenine dinucleotide as a coenzyme, mainly in tissues such as kidney, large intestine, placenta and testis expressed in microsomes. Microsomes containing 11β-HSD2 can be prepared according to conventional methods (Brennan, J. et al., Genes Dev, 2003.17, 800), and can also be purchased from reagent companies.
[0080] 11β-HSD2 enzyme activity was detected according to the existing research method (Haider, S.G.Int.Rev.Cytol., 2004, 233, 181). Briefly, reaction tubes were first labeled, and 40 μg of human kidney cell microsomal protein and 1 mM curcumin analog (dissolved in dimethylphenylsulfone, DMSO) were added to each tube, and then PBS was added to 100 μl. Then prepare steroid detection mixture: 2 μl [1,2- ...
Embodiment 3
[0088] Embodiment three: 7 kinds of curcumin analog preparations for the treatment of human colorectal cancer
[0089] Compounds YD1-YD7 prepared according to the method of Example 1 were respectively added with excipients according to the weight ratio of the excipients at a ratio of 5:1, and then granulated and compressed into tablets. For liquid preparations, compound YD1-YD7 can be prepared according to the method of Example 1, then add water for injection according to the conventional injection preparation method, and then make injection after fine filtration, potting and sterilization.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com