Novel crystalline forms of an inhibitor of 11-beta-hydroxysteroid dehydrogenase type 1
A crystalline, chlorophenyl technology is applied in the field of effective inhibitors of 11β-hydroxysterol dehydrogenase-1 enzyme, and can solve the problems of no disclosure and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
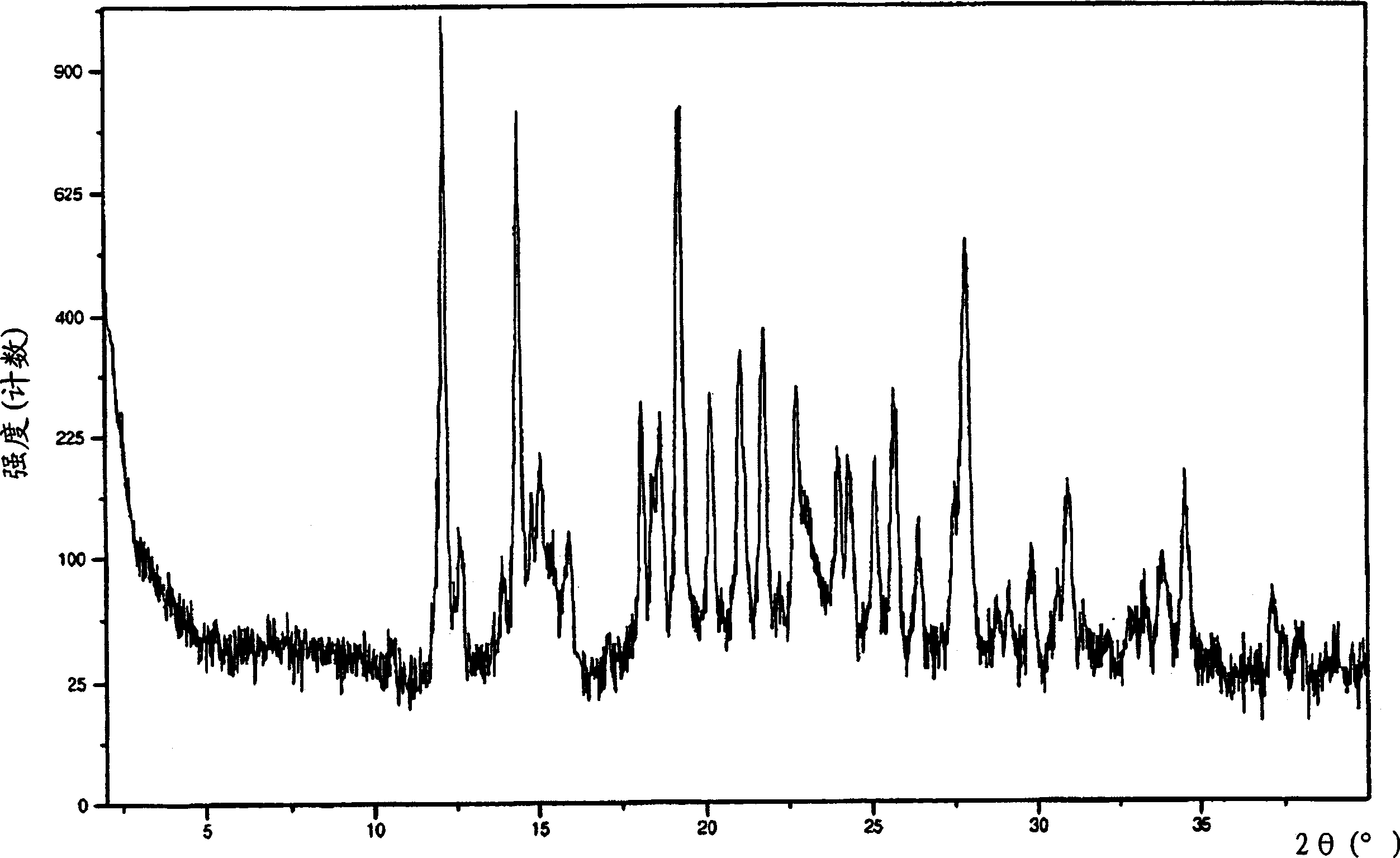
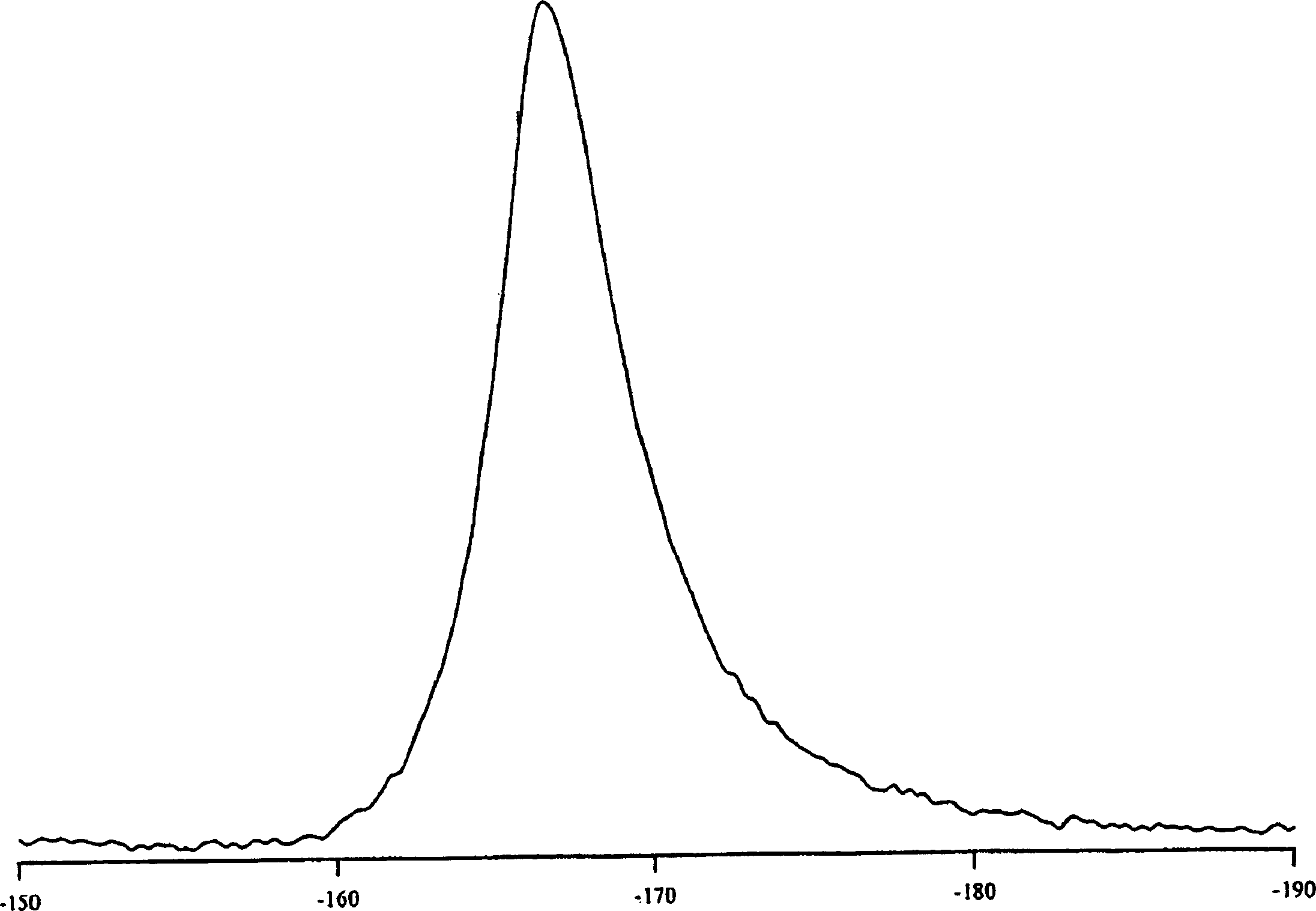
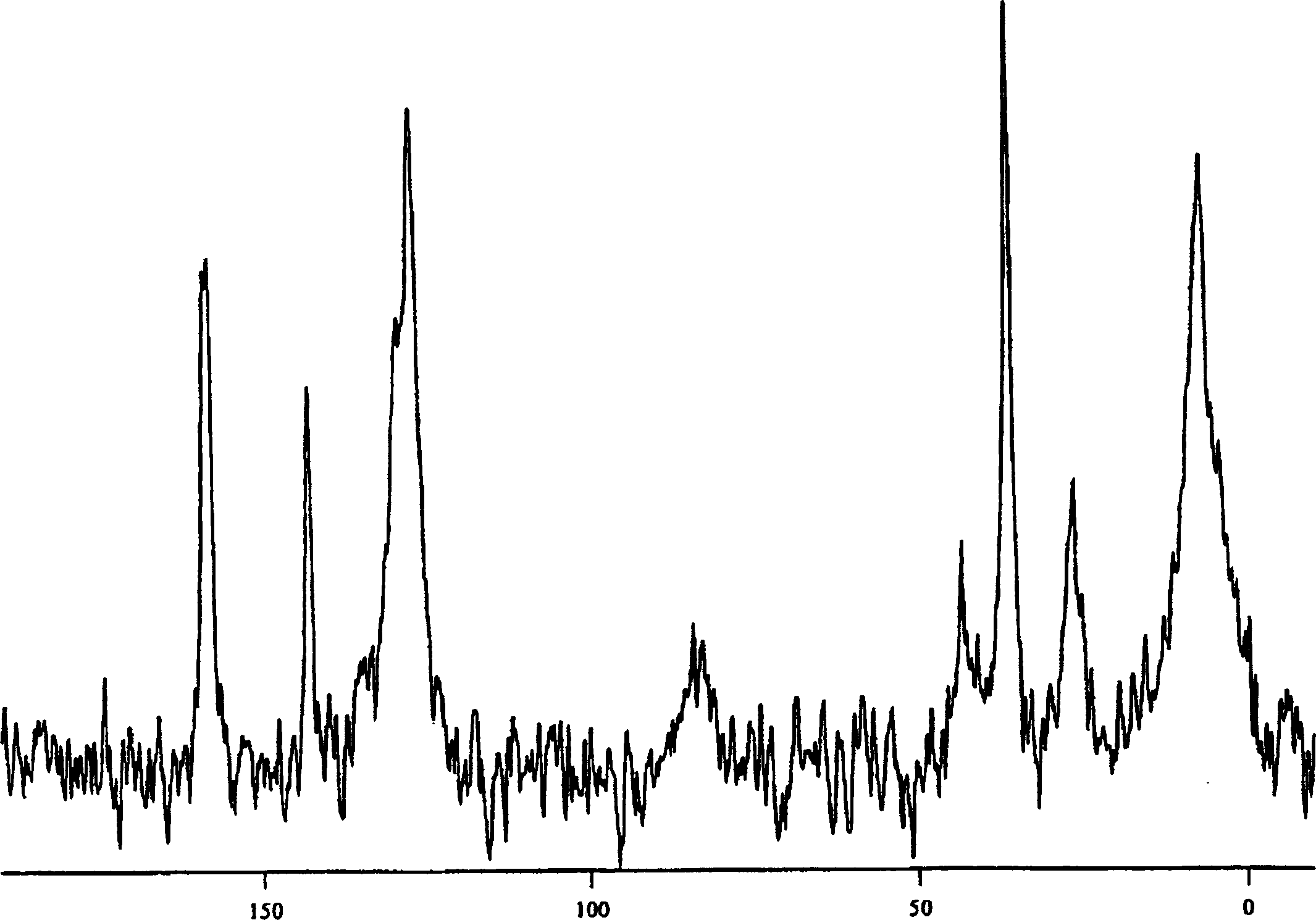
[0063] 3-[1-(4-Chlorophenyl)-trans-3-fluorocyclobutyl]-4,5-dicyclopropyl-r-4H-1,2,4-triazole crystalline anhydrate
[0064] Bisulfate 2-11 (39.0 g, 90.9 mmol) slurry in IPAc (150 mL) with 10% Na 2 CO 3 (100 mL) was mixed until all solids were dissolved. The aqueous layer was removed, and the organic layer was washed twice with 50 mL of water. The organic layer was then concentrated in vacuo to 70 g (ca. 45 mL residual IPAc). Some product crystallized out during concentration. Heptane (180 mL) was added slowly and the mixture was aged for 2 hours. The product was filtered and the filter cake was washed with heptane. Air drying followed by oven drying at 40°C gave the crystalline anhydrate free base as a white solid.
Embodiment 2
[0066] 3-[1-(4-Chlorophenyl)-trans-3-fluorocyclobutyl]-4,5-dicyclopropyl-r-4H-1,2,4-triazole crystalline anhydrate
[0067] bisulfate 2-11 (9.75 g, 22.7 mmol) in toluene (60 mL) was added water (60 mL) and 50% NaOH (4.54 g, 56.8 mmol). The mixture was stirred until all solids dissolved. Discard the aqueous layer. The organic layer was washed with 5% aqueous NaCl (60 mL). The organic layer was concentrated by vacuum distillation to about 38 mL. The solution was then transferred through an in-line filter to a stirred suspension of free base seeds (483 mg) in heptane (135 mL) over 3 hours. The resulting mixture was cooled to 0° C., stirred for 30 minutes, then filtered, washing with 4:1 heptane-toluene (30 mL) followed by heptane (30 mL). The crystalline material was dried under vacuum at 40°C to give the crystalline anhydrate form.
Embodiment 3
[0069] 3-[1-(4-Chlorophenyl)-trans-3-fluorocyclobutyl]-4,5-dicyclopropyl-r-4H-1,2,4-triazole crystalline monohydrate
[0070] to 6.58g bisulfate 2-11 To a slurry in IPA (15 mL) was added water (7.5 mL) and 10N NaOH (3.2 mL). The mixture was warmed to 40°C until all solids dissolved. The aqueous layer was separated and more water (7.5 mL) was added to the organic layer. Anhydrous free alkali seeds were introduced into the mixture and aged for 0.5 hours. More water (30 mL) was slowly added via syringe pump over 3 hours. The mixture was aged overnight and filtered. The filter cake was washed with 3:1 water / IPA (20 mL), then water (40 mL). Air drying overnight gave the crystalline monohydrate form.
[0071] 3-[1-(4-Chlorophenyl)-trans-3-fluorocyclobutyl]-4,5-dicyclopropyl-r-4H-1,2,4-triazole crystalline anhydrate
[0072] The monohydrate was then obtained in the crystalline anhydrate form by drying the monohydrate in a vacuum oven at 40°C with a slow flow of nitrogen.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com