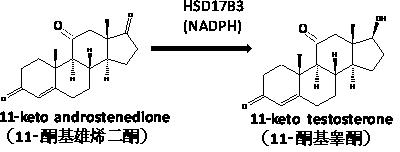
Medical application of 11-ketoandrostenedione and 11-ketotestosterone
A ketoandrostenedione and ketotestosterone technology, which can be applied to medical preparations containing active ingredients, pharmaceutical formulations, antitumor drugs, etc., can solve the problem of inability to reduce the concentration of cortisol in the whole body, and achieve good resistance to hypogonadism. Effects of sexual dysfunction and/or infertility, accelerated metabolism, relief of symptoms of hypogonadism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
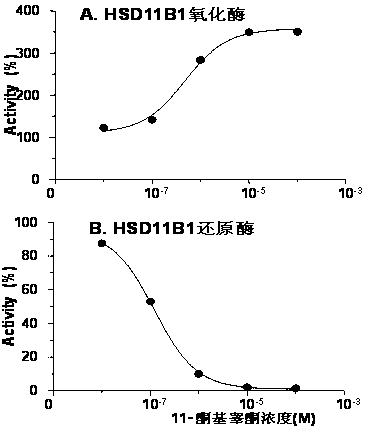
[0046] Example 1: 11-ketotestosterone dually regulates HSD11B1 activity of rat Leydig cells in vitro
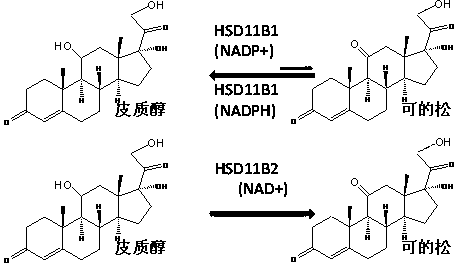
[0047] 1. HSD11B1 is an oxidoreductase with NADP+ / NADPH as coenzyme, mainly expressed in Leydig cells. The measurement of HSD11B1 activity in Leydig cells does not require the addition of NADP+ / NADPH. Compounds were measured with intact Leydig cells, reflecting compound penetration into cells.
[0048] 2. Isolation of Leydig cells from adult rat testes: The testes of 6 SD rats aged 90 days were used for the separation of Leydig cells from adult rats. The separation method was prepared according to the method reported by Salva, et al [Salva, A.; Klinefelter, G.R.; Hardy, M.P.J.Androl. 2001, 22, 665]. The purity of Leydig cells was determined according to the method reported by Payne et al [Payne. Endocrinology, 1980]. Leydig cells were 95% pure as assessed by histochemical staining for 3b-steroid dehydrogenase.
[0049] 3. Activity detection of HSD11B1 oxidation and reduct...
Embodiment 2
[0051] Example 2: 11-ketoandrogen dual regulation of human HSD11B1 activity in vitro
[0052] 1. Human HSD11B1 enzyme can be produced by HSD11B1 gene expression and COS1 cell line. The measurement of HSD11B1 activity in COS1 cells does not require the addition of NADP+ / NADPH. Compounds were measured with intact COS1 cells, reflecting compound penetration into cells.
[0053] 2. Human HSD11B1 enzyme comes from HSD11B1 gene expression: Human HSD11B1 gene was transfected into COS1 cell line and cultured in COS1 cell line: according to Ge et al (Ge et al 2000. J Androl. 21(2):303-10.) Human HSD11B1 gene transfected and cultured CHOP cell line.
[0054] 3. Activity detection of HSD11B1 oxidation and reduction in COS1 cell line: The activity detection of COS1 cell line HSD11B1 was carried out according to the method reported by Ge RS, et al. [Ge RS, et al. Endocrinology, 1997, 139 (9): 3787-95 ]. Using [3H]-cortisol as a substrate, the amount of cortisone produced was detected t...
Embodiment 3
[0056] Example 3: Mode of action of 11-ketoandrogen regulating HSD11B1 activity in vitro
[0057] 1. HSD11B1 is an oxidoreductase with NADP+ / NADPH as coenzyme, mainly expressed in hepatocytes. HSD11B1 activity in hepatocyte microsomes is affected by hexose phosphate dehydrogenase and so on. Hexose phosphate dehydrogenase uses hexose phosphate (G6P) as a substrate to generate NADPH to stimulate HSD11B1 reductase activity and reduce HSD11B1 oxidase activity. The measurement needs to add NADP+ or G6P.
[0058] 2. Rat hepatocyte microsomes were prepared according to the method reported by Ge RS, et al [Ge RS, et al. Endocrinology, 1997, 139(9):3787-95].
[0059] 3. Activity detection of HSD11B1 oxidation and reduction in rat hepatic microsomes: The activity detection of HSD11B1 in hepatic microsomes was carried out according to the method reported by Ge RS, et al. Endocrinology, 1997, 139 (9 ):3787-95]. Using [3H]-corticosterone as a substrate, the amount of 11-dehydrocorticos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com