Methods for producing dodecanedioic acid and derivatives thereof
A technology of dodecanedioic acid and dodecanedioic acid, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylate, etc., and can solve problems such as price fluctuations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
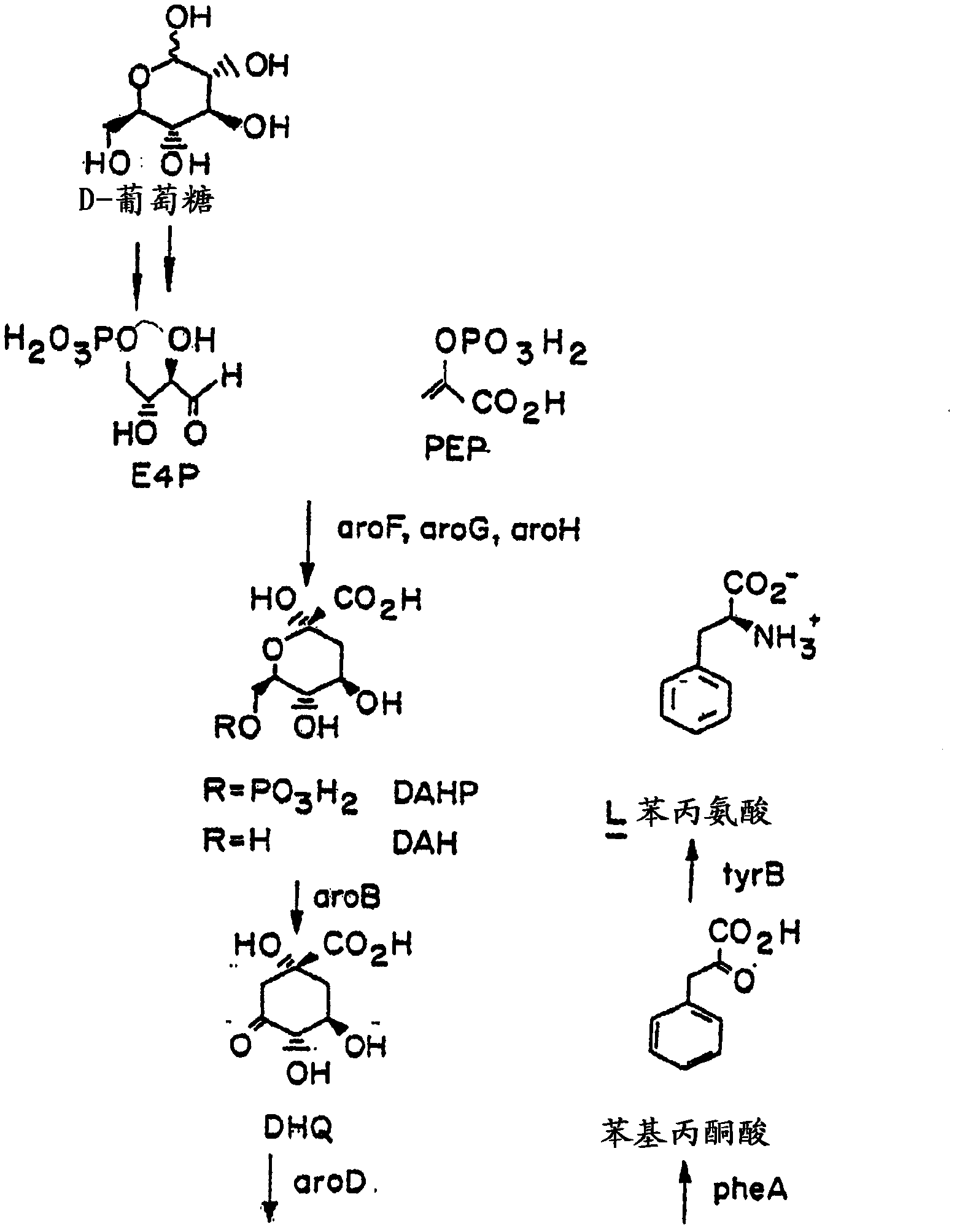
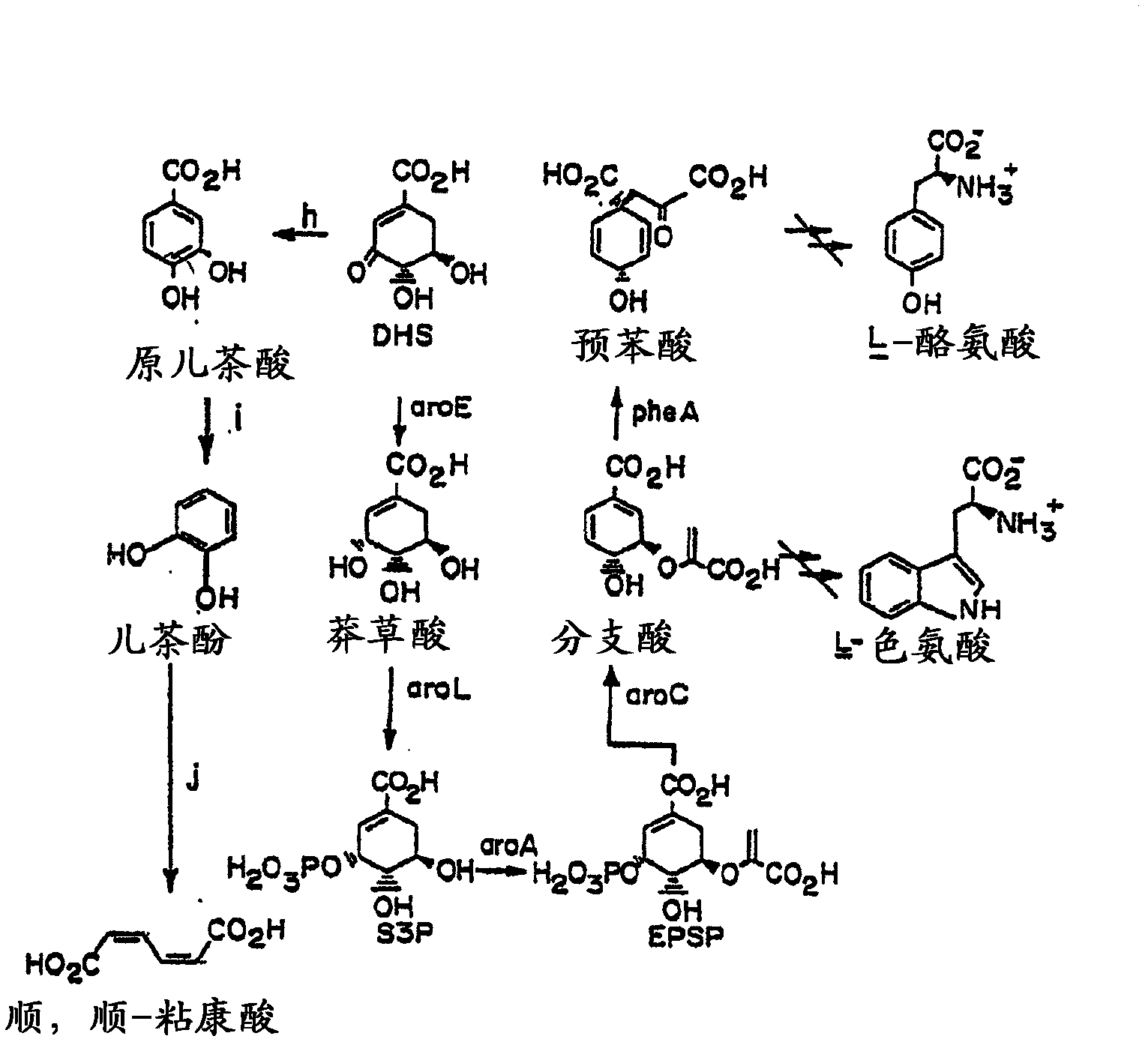
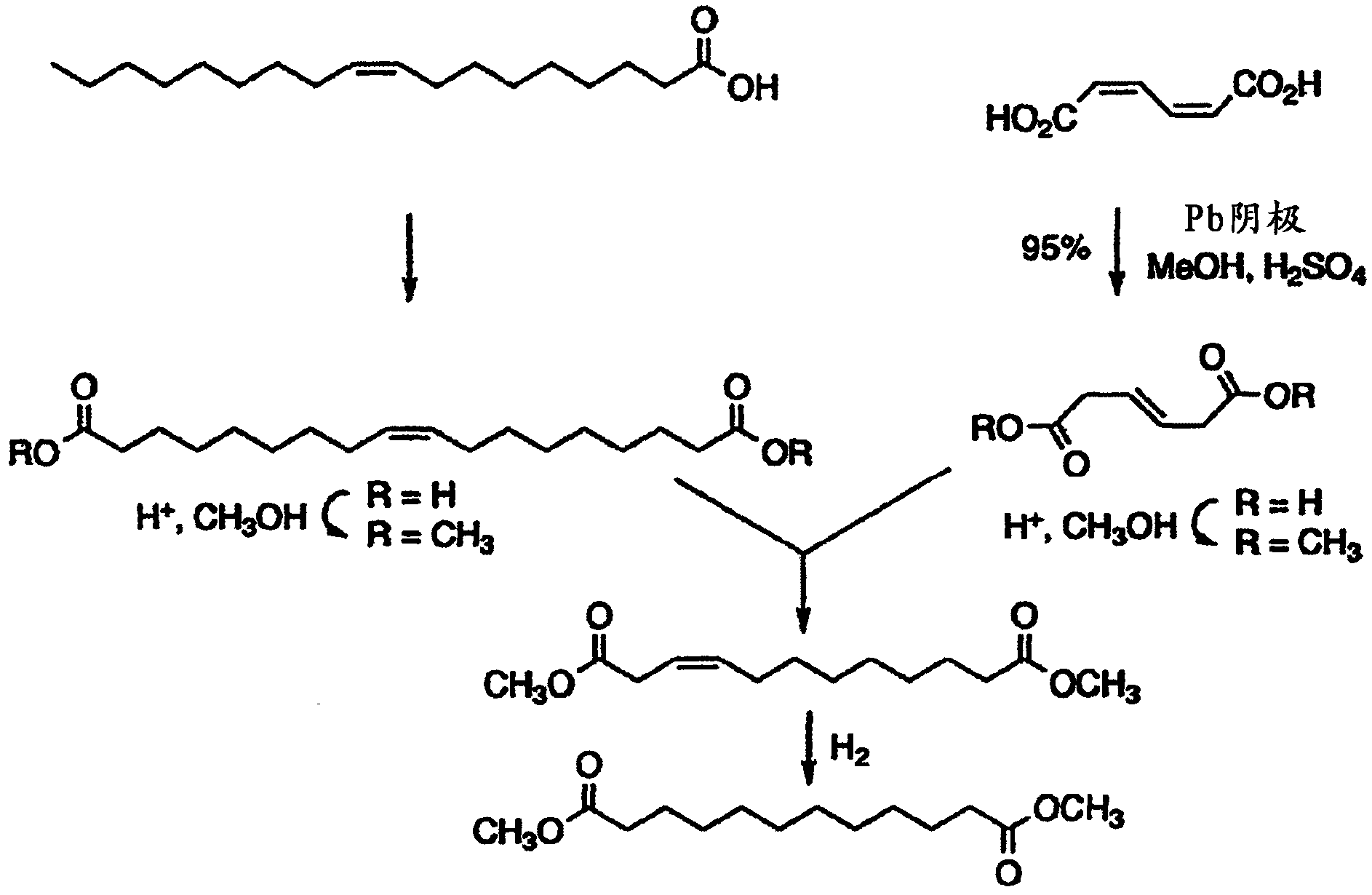
[0026] The present invention relates to methods and compositions for producing dodecanedioic acid and its derivatives and / or dodecanedioic acid and its derivatives. In certain aspects, the present invention relates to methods and compositions for generating dodecenedioic acid from 3-hexenedioic acid and octadecenedioic acid ester. In a preferred embodiment, dodecenedioic acid yields dimethyl adenoate and dimethyl octadecenoate. In a preferred embodiment, reduction of dimethyldodecanedioic acid produces dimethyldodecanedioic acid. As used herein, the terms hexenedioate and hexenedioate are used interchangeably and mean a compound containing 6 carbon atoms, 8 hydrogen atoms and 4 oxygen atoms and having the molecular formula HOOC-CH 2 -CH=CH-CH 2 Molecule of -COOH. As used herein the terms dodecenedioate and dodecenedioate are used interchangeably and mean a compound containing 12 carbon atoms, 20 hydrogen atoms and 4 oxygen atoms and having the molecular formula HOOC-CH 2 -...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com