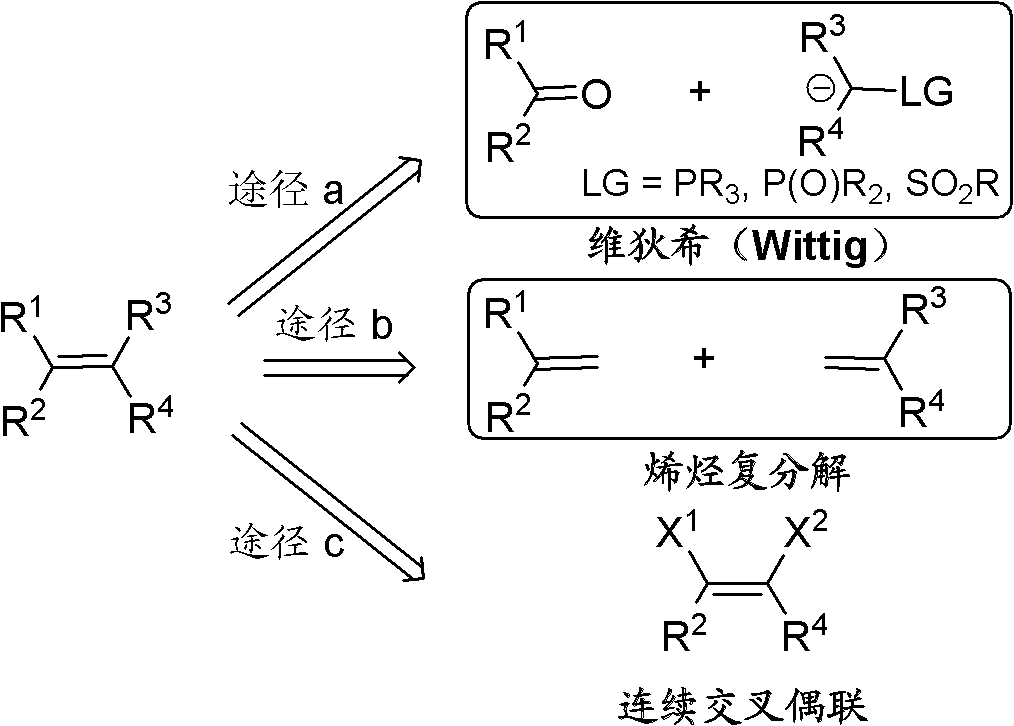
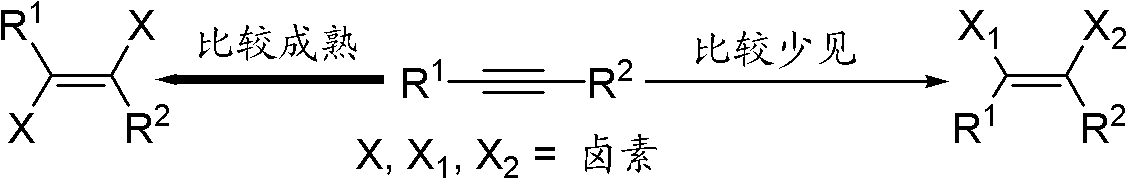
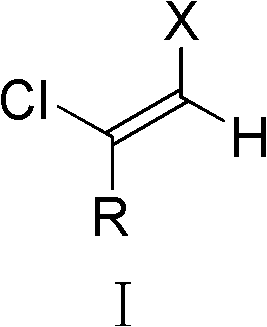
Cis-1-halo-2-chloroalkene and preparation method and application thereof
A chlorinated olefin, cis technology, applied in the field of cis-1-halo-2-chloroalkene and its preparation, can solve problems such as powerlessness, reduce environmental pollution, reduce "three wastes" emissions, economic benefits and social significant effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051]Lithium chloride (26mg, 0.6mmol) and palladium acetate (5.6mg, 0.025mmol) were added in a 10mL round bottom flask, 1mL of acetic acid was added to dissolve, then phenylacetylene chloride (73mg, 0.5mmol) and Allenyl ester (17.4 mg, 0.1 mmol). The reaction system was reacted at 60° C. for 5 hours, quenched by adding 5 mL of water, and extracted three times with ethyl acetate (10 mL). After the combined organic phases were washed with sodium bicarbonate solution and saturated brine, the organic layer obtained was dried over anhydrous sodium sulfate, and then the solvent was removed by rotary evaporation, and then separated by silica gel (300-400 mesh) column chromatography (eluent: petroleum ether) to obtain 71 mg of cis-1,2-dichlorostyrene (yield: 82%) as a colorless liquid.
[0052] colorless liquid 1 H-NMR (CDCl 3 , 400MHz): δ6.70(s, 1H), 7.38-7.40(m, 3H), 7.53-7.55(m, 2H); 13 C-NMR (CDCl 3 , 100MHz): δ116.1, 126.6(2C), 128.7(2C), 129.5, 135.6, 135.8; MS(EI, m / z): 1...
Embodiment 2
[0055] Except that the palladium acetate of 0.01 mmol is used to replace the palladium acetate of 0.025 mmol in Example 1 as the catalyst, all the other operating steps are the same as in Example 1 to obtain cis-1,2-dichlorostyrene with a yield of 81%, product characterization data With embodiment 1.
Embodiment 3
[0057] Except that the phenylacetylene chloride in Example 1 was replaced by 4-fluorophenylacetylene chloride, the remaining operation steps were the same as in Example 1, yield: 68%, product: colorless liquid. colorless liquid 1 H-NMR (CDCl 3 , 400MHz): δ6.65(s, 1H), 7.08(t, J=8.4Hz, 2H), 7.50-7.54(m, 2H); 13 C-NMR (CDCl 3 , 100MHz): δ115.7(d, J=21.9Hz, 2C), 115.9(d, J=1.6Hz, 1C), 128.5(d, J=8.4Hz, 2C), 132.0(d, J=3.3Hz , 1C), 134.6, 162.8 (d, J=248.6Hz, 1C); MS (EI, m / z): 194(6), 192(38), 190(M + , 58), 157(32), 155(M + - 35 Cl, 100). It shows that the colorless liquid is cis-1,2-dichloro-2-p-fluorostyrene represented by structural formula 3b.
[0058]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com