p28GANK monoclonal antibody and application thereof
A monoclonal antibody and hapten technology, applied in the field of medical bioengineering, can solve the problem of unclear progress of liver cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1. Anti-human p28 GANK Preparation and purification of monoclonal antibodies
[0030] 1. Antigen Synthesis and Coupling
[0031] The synthetic design of antigenic polypeptides refers to GenBank, GeneID: 5716, and the specific sequences are NPDAKDHYEATAMHRC (SEQ ID NO: 1), CDEERVEEAKLLVSQ (SEQ ID NO: 2), YIENKEEKTPLQVAKGC (SEQ ID NO: 3), provided by Pocky Biotechnology (Shanghai) Co., Ltd. The company uses a solid-phase peptide synthesis method to synthesize peptides through an automatic peptide synthesizer.
[0032] The coupling of peptides and keyhole limpet hemocyanin (KLH) adopts the glutaraldehyde linkage described on page 865 of "Molecular Cloning Experiment Guide" 2nd Edition, written by Sambrook, translated by Jin Dongyan, Science Press, Beijing, 1999, p. Law.
[0033] 2. Preparation and Purification of Monoclonal Antibodies
[0034] 100 μg of the polypeptide KLH conjugate purified in the above 1 was diluted with PBS, mixed with an equal volume of com...
Embodiment 2
[0038] Example 2. Anti-human p28 GANK Identification and detection applications of monoclonal antibodies
[0039] 1. Western Blot detection application of monoclonal antibody
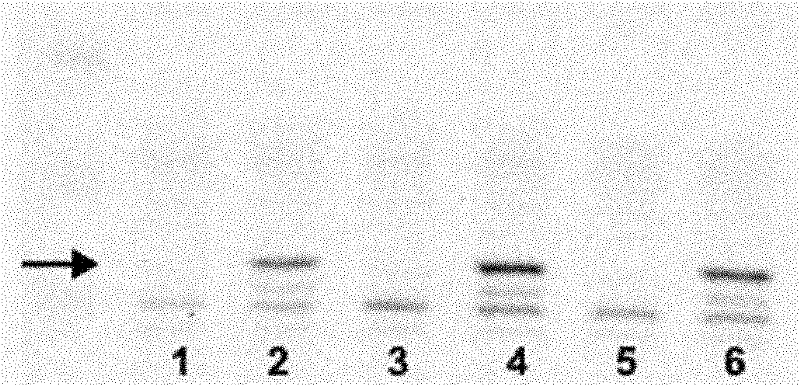
[0040] The denatured protein sample was subjected to SDS-PAGE protein electrophoresis; the protein was transferred to a nitrocellulose membrane (Schleicher & Schell company) by an electroporator; TBST containing 5% BSA was blocked for 1 h, washed once in TBST for 5 min; TBST containing 4% BSA was diluted with anti p28 GANK Monoclonal antibody primary antibody (1μg / ml), incubate for 2h, wash three times with TBST, 5min each time; dilute IR-800-labeled anti-mouse secondary antibody in TBST containing 4% BSA, incubate for 1h, wash four times with TBST, each time 5min; scan with an infrared laser scanner Odyssey. see results figure 1 . The results show that the monoclonal antibody can specifically recognize human p28 GANK molecular.
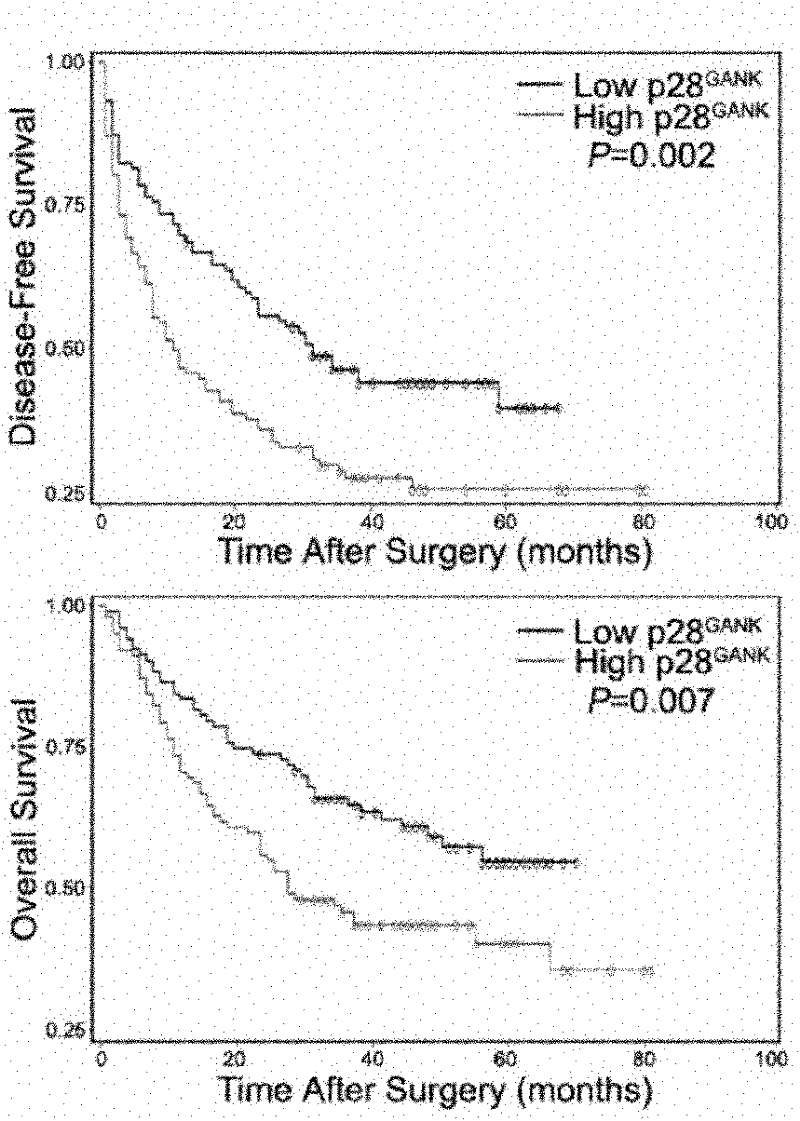
[0041] 2. Application of immunohistochemical detection of monoclon...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap