Spectinomycinamide as an anti-tuberculosis agent
A halogen, compound technology, applied in the field of spectinomycin amide as an anti-tuberculosis agent, can solve the problems of patient non-compliance, increased number, limited treatment options, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0792] Synthesis of Spectinomycin Analogs
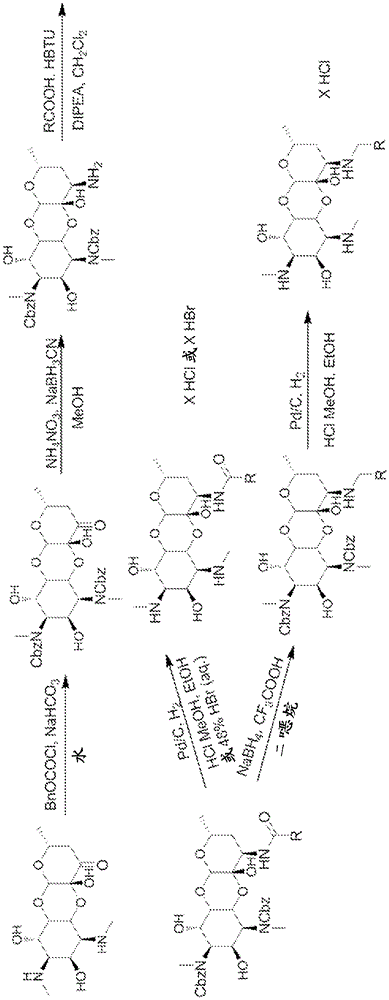
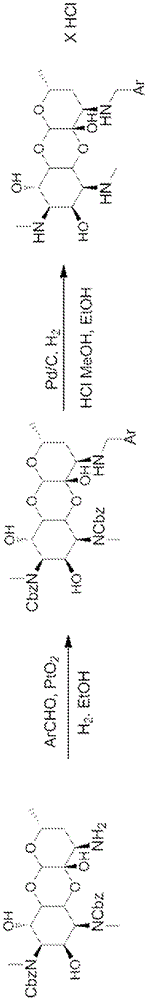
[0793] General Methods: The 3'-deoxy3'-acylamino and 3'-deoxy3'(R)-alkylamino spectinomycins disclosed in the present invention were synthesized according to methods similar to those previously described. see Woitun et al. J. Antibiot (Tokyo), 34(1), 22-27(1981); and Maier et al. , J. Antibiot (Tokyo), 34(1), 16-21 (1981). 1,3-Dibenzyloxycarbonyl-3′(R)-aminospectinomycin from spectinomycin dihydrochloride pentahydrate as figure 1 The synthesis shown is in two steps. First, apply benzyl chloroformate and NaHCO in water 3 , the methyl secondary amine in ring A was protected as benzyl carbamate with carboxybenzyl (CBz). The protected intermediate was then reductively aminated with ammonium nitrate and sodium cyanoborohydride in methanol to afford the 3'-deoxy-3'-amino derivative.
[0794] The amines were then used in the synthesis of the target 3'-acylaminospectinomycin derivatives by coupling them to various acids by using H...
Embodiment 2
[0870] General in vitro and in vivo methods
[0871] MIC determination: application according to Clinical Laboratory Standards Institute (ClinicalLaboratoryStandardsInstitute) (CLSI; National, C.F.C.L.S., Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically-Seventh Edition: Approved Standard M7-A7, CLSI, Wayne, Pennsylvania, U.S.A., 2008) was used to measure the MIC by a microbroth dilution method, and the reading was checked visually. 2-fold serial dilutions of antibiotics in 100 μL of appropriate broth medium were first prepared in 96-well round bottom microtiter plates (NalgeNunc International, Rochester, New York, USA). Add an equal volume (100 μL) of about 10 5 Bacterial broth inoculum in cfu / mL of bacteria to give a final drug concentration starting at 200 μg / mL and the plates were incubated aerobically at 37°C. M. tuberculosis and M. bovis BCG microtiter plates were incubated for 7 days, while the other strains were incubated o...
Embodiment 3
[0883] Antituberculous activity
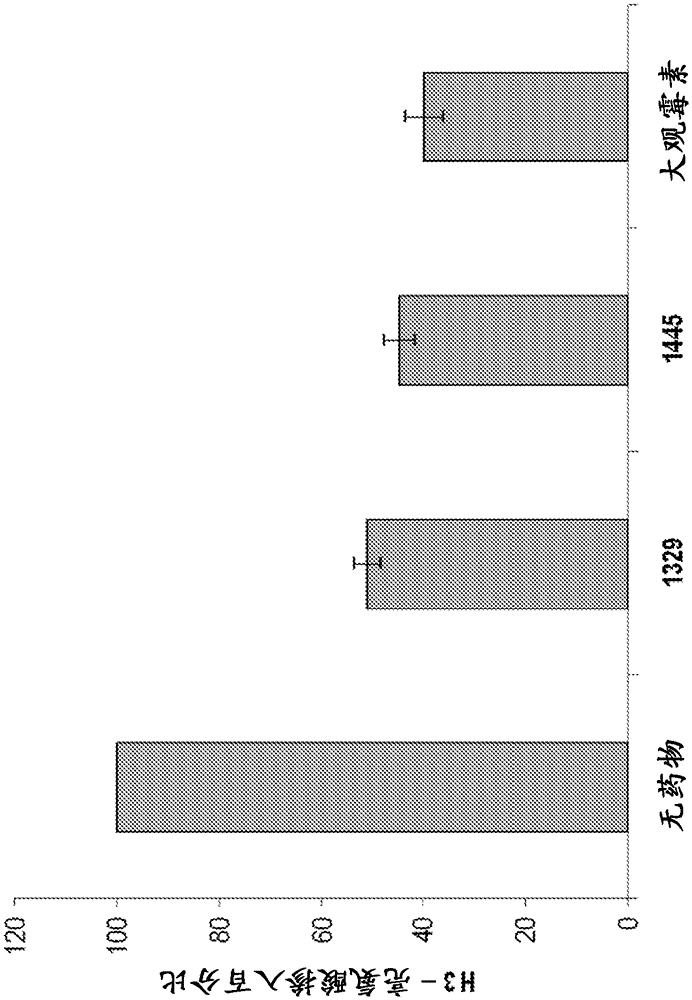
[0884] The anti-tuberculosis activity of spectinomycin analogs against Mycobacterium tuberculosis H37Rv was determined in Middlebrook 7H9 supplemented with 10% ADC medium by dilution in drug microbroth in 96-well plates. Plates were incubated at 37°C for 7 days and then read visually for growth inhibition according to a previously described method. See for example Hurdle et al. , J. Antimicrob. Chemother, 62(5), 1037-1045 (2008). The results are shown in Table 1 and Table 2.
[0885] Table 1.3'-deoxy3'(R)-acylamino spectinomycin anti-tuberculosis activity
[0886]
[0887]
[0888]
[0889]
[0890] Table 2.3'-Deoxy 3'(R)-Alkylamino Spectinomycin Anti-tuberculosis Activity
[0891]
[0892] Multiple compounds showed good anti-tuberculosis MIC values, many of which had better anti-tuberculosis activity compared to spectinomycin. The structure-activity relationship of this series is very tight in terms of structural change...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com